Translate this page into:
Synthesis, characterization, anticancer activity assessment and apoptosis signaling of fucoidan mediated copper oxide nanoparticles
⁎Corresponding author at: Department of Food Science and Biotechnology, College of BioNanotechnology, Gachon University, 1342 Seongnam-daero, Gyeonggi-do 13120, Republic of Korea. hyejinp@gachon.ac.kr (Hye-Jin Park)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nanotechnology is expanding at a very fast rate owing to its many possible applications in the industrial, biomedical, commercial, and other areas. In this study, copper oxide nanoparticles (CuO-Fu-NPs) were prepared using Undaria pinnatifida-derived fucoidan as a capping and reducing agent, and their anticancer activity and apoptosis-related mechanism were assessed using HeLa cells. Fourier-transform infrared spectroscopy, field emission scanning electron microscopy, energy dispersive X-ray spectroscopy, and X-ray powder diffraction were used to characterize the fabricated product, revealing that the fucoidan molecules had reduced the Cu ions into NPs and coated the CuO NP surface. Circular NPs of <30 nm in size were also trapped in fucoidan sheets. The CuO-Fu-NPs inhibited HeLa cell growth significantly in a dose- and time-dependent manner, with an IC50 value of 0.479 mg/mL (compared with 1.104 mg/mL with fucoidan alone). According to TUNEL assay results, the CuO-Fu-NPs induced DNA damage and apoptosis in the HeLa cells. Western blot analysis revealed the expression of apoptosis-associated proteins (BCL2, BAX, cytochrome c, cleaved CASP-3 (cleaved caspase-3), and cleaved PARP) in the CuO-Fu-NPs-treated cells. This study provides valuable information about the toxic effect of this biomaterial, which could have biomedical applications.
Keywords
Fucoidan
Nanoparticles
Anticancer
Apoptosis
HeLa cells
- CuO-Fu-NPs
-
Copper oxide nanoparticles capped with Undaria pinnatifida-derived fucoidan
- CuO
-
copper oxide
- Nanoparticles
-
NPs
- cleaved CASP-3
-
cleaved caspase-3
Abbreviations
1 Introduction
Nanotechnology has led to the integration of a diverse array of disciplines in the biomedical, chemical, and industrial areas (Jian et al., 2020), with many different nanomaterials being used in various fields, most importantly in medicine, diagnostics, drug delivery, and therapeutics (Faraji and Wipf, 2009; Saji et al., 2010). Nanomaterials are advantageous because of their small size and good biocompatibility. Therefore, metallic, inorganic, and organic nanomaterials are commonly used in nanotechnology (Liu et al., 2016).
Seaweeds (marine algae) and their products are widely consumed by the populations of China, Korea, and Japan, with Undaria pinnatifida (a brown seaweed species commonly known as wakame or mekabu) being especially popular for its nutritionally and pharmacologically important biocompounds. U. pinnatifida consists of various polysaccharides, such as cellulose, alginates, and the sulfated polysaccharide fucoidan (Fig. 1) (Phull and Kim, 2017, 2018). Various potential bioactivities of fucoidan have been reported, including its antimicrobial, anticancer, anti-inflammatory, antitumor, anticoagulation, antioxidative, and immunomodulatory effects (Phull et al., 2017b; Synytsya et al., 2010; Wang et al., 2010). Moreover, fucoidan has been shown to induce apoptosis in lymphoma cells (Park et al., 2011), gastric carcinoma cells (Haneji et al., 2005), and breast carcinoma cells (Yamasaki-Miyamoto et al., 2009). Hence, given that it is a reservoir of natural bioactive compounds, fucoidan would be useful as a reducing and capping agent for the production of biofunctional nanoparticles (NPs).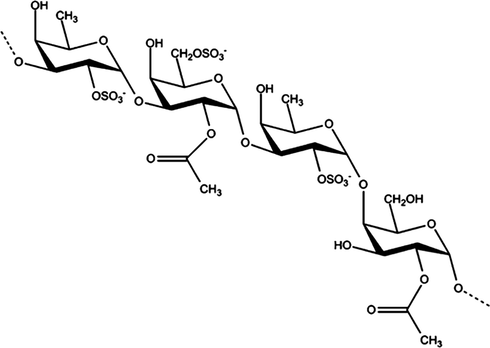
Structure of fucoidan.
Among the inorganic metallic particles that can be applied for the production of nanomaterials, copper oxide (CuO) NPs are widely used owing to their intrinsic electrical, mechanical, optical, and catalytic properties (Arasu et al., 2019; Govindaraju et al., 2020; Ramu et al., 2020). Importantly, the ready and inexpensive availability of CuO NPs is the main reason for their wide use in modern applications of nanoproducts (Mallakpour et al., 2020).
Traditional methods of synthesizing nanoparticles, like chemical and physical methods. The chemical method of synthesis includes chemical reduction, electrochemical techniques and photochemical reduction. The classical chemical method in which a reducing agent such as sodium borohydride and hydrazine as well as radiation chemical method generated by ionization radiation are also used for chemical synthesis of nanoparticles. The physical methods of synthesis include condensation, evaporation and laser ablation. These methods are expensive, inefficient and can release harmful wastes to the environment hence a better eco-friendly methods are preferred.
Green synthesis is an emerging area in the field of bio-nanotechnology and provides economic and environmental benefits as an alternative to chemical and physical methods. In this method nanoparticles involves nontoxic safe reagents which are eco-friendly and biosafe material is used. Various natural resources available in nature such as plant extracts, algae, microbes or their derived substances, for instances polysaccharides including fucoidan, chitosan, and many more could be used for the green synthesis of metal oxide nanoparticles (Keabadile et al., 2020; Pandit and Gayatri, 2020)
Thus, the synthesis of Cu NPs with seaweed-derived components can be carried out economically and on a commercial scale (Ganesan et al., 2020; Kannan and Sundrarajan, 2015). Because seaweeds are natural products, any anticancer effect they have would not impose toxicity onto normal cells (Cunha and Grenha, 2016). Capping agents prominently involved in the characteristic modification of the nanoparticles which makes them attractive agent for biomedical applications including anticancer and as drug delivery component. The biological functions and alleviated adverse effects of NPs are demarcated by their chemical features such as surface chemistry, shape, and size which is accredited towards the appropriate capping agent. NPs associated toxicity and safety concerns are increasing because of their long term usage in the biological system. However, capping with natural material like fucoidan, plant extract, etc. is an effective way for the production of nanoparticles with less toxicity. Therefore, NPs produced using seaweed-derived compounds would be reliable, harmless, economical, and eco-friendly in nature compared with those prepared through physical and chemical means.
The design and synthesis of metallic NPs of specific particle sizes are of great importance for their application as nanomedicines for anticancer, antimicrobial, anti-inflammatory, and antiangiogenic treatments (Chen et al., 2021; Phull et al., 2016, 2020). In this study, CuO NPs were produced using U. pinnatifida-derived fucoidan as a reducing and capping agent. The synthesized CuO-Fu-NPs were first characterized and then used to treat a human cervical cancer (HeLa) cell line to study their anticancer and toxic effects.
2 Materials and methods
2.1 Cancer cells, reagents, and chemicals
The HeLa cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). Primary antibodies against tumor protein 53 (p53), B-cell lymphoma 2 (BCL2), apoptosis regulator BAX (BAX), cytochrome c, caspase-3, cleaved CASP-3, poly [ADP-ribose] polymerase (PARP), cleaved PARP, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase-conjugated secondary biotinylated antibodies were obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Gibco (Carlsbad, CA, USA). Streptomycin, penicillin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the material used in the experiment was either autoclaved or sterilized. All the procedures were carried out in the sterilized conditions. Unless otherwise stated, all other chemicals and reagents used were obtained from Sigma-Aldrich.
2.2 Synthesis of CuO-Fu-NPs
CuO NPs were synthesized using U. pinnatifida-derived fucoidan according to a previously reported method (Naz et al., 2020) with slight modifications. Fucoidan of Undaria pinnatifida (HWED-15042001), was obtained from Haewon Biotech, Inc. Republic of Korea. First, an aqueous solution of fucoidan (1%) was warmed for 5 min, following which copper(II) acetate monohydrate (0.5%) was added. The mixture was placed on a hot plate set at 55 °C and allowed to react for 2 h with continuous stirring. A color change from greenish brown to dark brown indicated the synthesis of NPs, which were then isolated by centrifugation (Ependorf Centrifuge 804) at 10,000 rpm for 10 min. After three washes with distilled deionized water, the NPs were dried in an oven at 60 °C for 5 h. The dried powder was collected for characterization and biological testing.
2.3 Characterization of the sample
The formation of the CuO NPs and their capping with fucoidan were assessed by Fourier-transform infrared (FTIR) spectroscopy, X-ray powder diffraction (XRD), and field emission scanning electron microscopy (FE-SEM). The FTIR analysis was performed using a Shimadzu FTIR apparatus (Shimadzu, Kyoto, Japan), with recording in the wavelength range of 400–4000 cm−1. The crystallographic planes of the prepared CuO-Fu-NPs were analyzed by XRD using an X’Pert3 Powder system (Malvern Panalytical, Malvern, UK), operating at 40 kV voltage and 30 mA current at ambient temperature. The nickel monochromator had an angle of diffraction (2θ) in the range of 20–80° with a Cu Kα radiation source of wavelength 1.5406 Å. The sample for the characterization of SEM analysis was prepared by adopting air drying method. The aqueous suspension of prepared nanoparticles was successively air dried on the glass substrate in order to have a uniform film of it and then that sample was further analyzed for SEM analysis using a JEOL-JSM-6490LA scanning electron microscope (JEOL, Tokyo, Japan) operating at 20 kV with 2838 counts per second.
2.4 Anticancer assessment
All the anticancer effects of the synthesized CuO-Fu-NPs and of fucoidan alone were evaluated using MTT and terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assays, as described below.
2.4.1 Antiproliferative/viability activity against cervical cancer (HeLa) cells
2.4.1.1 Culture of HeLa cells
HeLa cells were maintained in DMEM containing 10% heat-inactivated FBS, streptomycin (50 µg/mL), and penicillin (50 U/mL). For the TUNEL assay, cells at a density of 2.5 × 104/well were grown on glass coverslips in 24-well culture plates. For the MTT assay, 1.5 × 103 cells/well were grown in 96-well plates. For both cultures, incubation was carried out under a humidified atmosphere in an incubator (95% O2, 5% CO2) at 37 °C. The medium was changed after 2 days and the assays were conducted when the cells had reached 70–75% confluence. The particles were weighed and suspended in phosphate-buffered saline (mg/ml) followed by sonication for 30 min for complete dispersion of NPs in the medium and desired contraction was exposed to the cells for MTT and TUNEL bioassays.
2.4.1.2 MTT assay
The toxic effects of the CuO-Fu-NPs and of fucoidan alone on the viability of HeLa cells were investigated using the MTT assay, as described previously [17]. In brief, HeLa cells were grown to 70–75% confluency in 96-well plates (as described in Section 2.4.1.1) and then exposed to various concentrations of fucoidan or CuO-Fu-NPs (0–4 mg/mL) for 24 h under 5% CO2 in an incubator at 37 °C. After the incubation period, 10 μL of MTT reagent (0.5 mg/mL) was added to each well. The plate was incubated under 5% CO2 in the incubator at 37 °C for 4 h until formazan crystals had formed in the wells. To dissolve the formazan crystals, 100 μL of a solubilization buffer [10% sodium dodecyl sulfate (SDS) with 0.01 N HCl] was added to each well and the plate was incubated for approximately 10 h in the CO2 incubator. Finally, the absorbance was recorded at 570 nm using a microplate reader (Tecan, Männedorf, Switzerland). Cells that had not been exposed to the test materials were considered to be 100% viable.
2.4.2 TUNEL assay
The TUNEL assay was used to investigate the effects of the synthesized particles on the HeLa cells at the molecular level. To identify DNA destruction, TUNEL staining using a commercially available kit (Roche Molecular Biochemicals, Mannheim, Germany) was carried out according to the manufacturer’s instructions. In brief, HeLa cells were grown on glass coverslips in 24-well plates (as described in Section 2.4.1.1) and then treated with CuO-Fu-NPs or fucoidan for 24 h in CO2 incubator. Then, after washing the cells with phosphate-buffered saline (PBS) to remove cell debris, they were fixed with 4% paraformaldehyde at ambient temperature for 15 min. Thereafter, the cells were washed with PBS and permeabilized with 0.1% Triton X-100 (in 0.1% sodium citrate) on ice for 2 min. Subsequently, the cells were washed with PBS and then incubated with the terminal deoxynucleotidyl transferase and rhodamine-dUTP solution at 37 °C for 1 h. Finally, after washing the cells with PBS, they were mounted using the ProLong Gold Antifade reagent containing the nuclear staining dye 4ʹ,6-diamidino-2-phenylindole, dihydrochloride (DAPI). Images were visualized and captured using a fluorescence microscope.
2.5 Western blot assay of anticancer- and apoptosis-related proteins
The western blot assay was used to investigate the effects of CuO-Fu-NPs and of fucoidan alone on the expression of proteins associated with cancer cell viability. First, control (non-treated) cells and cells treated with 2 mg/mL of the test material for 24 h were established and then harvested. For protein extraction, whole-cell lysates were prepared in radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Nonidet-40, a protease inhibitor cocktail (10 μg/mL of pepstatin A, leupeptin, and aprotinin and 1 mM 4–(2–aminoethyl) benzenesulfonyl fluoride), and phosphatase inhibitors (1 mM Na3VO4 and NaF). The crude cellular proteins were quantified by bicinchoninic acid assay using a standard curve of bovine serum albumin. Identical quantities of crude proteins were then size-fractionated by SDS-polyacrylamide gel electrophoresis. Thereafter, the separated protein bands were transferred to nitrocellulose membranes, which were then blocked with a blocking buffer [5% non-fat dry milk in Tris-buffered saline/Tween-20 (TBST)] for 1 h, washed with TBST, and probed with the primary antibodies (described in Section 2.1) at 4 °C for 14 h. Then, after washing the membranes with TBST, they were incubated with horseradish peroxidase-conjugated secondary antibodies (described in Section 2.1) for 2 h. Subsequently, the membranes were washed with TBST and developed with the EZ-Western Detection Kit (DaeilLab service, Seoul, Korea). Images of the protein bands were captured using a Fuji LAS-4000 imager (Fuji Film Co., Tokyo, Japan), and the densitometry of the blots was analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.6 Statistical analysis
All experiments were repeated three times and results are presented as the mean ± SD. Differences were considered statistically significant at p < 0.05. GraphPad Prism statistical software (GraphPad Prism, version 5.01; GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses of the data.
3 Results and discussion
3.1 Characterization of the synthesized CuO-Fu-NPs
FTIR analysis was carried out to investigate the formation of CuO NPs capped with fucoidan. Fig. 2 shows the FTIR spectra of fucoidan (Fig. 2A) and the CuO-Fu-NPs (Fig. 2B). Fucoidan has a complex structure, with hydroxyl and sulfate as the main functional groups. Its spectrum (Fig. 2 spectrum A) includes bands at 3389 and 1220 cm−1, which are assigned to the O-H and O-SO3H stretching modes of its functional groups. Metal oxide peaks in FTIR were found at 435, 505 and 594 cm−1. These stretching frequencies of fucoidan had deviated in the synthesized NPs (Fig. 2, spectrum B), where the stretching of O-H shifted from 3389 cm−1 to 3377 cm−1 and that of O-SO3H from 1220 cm−1 to 1230 cm−1, clearly indicating the involvement of these functional groups in the fucoidan capping of the CuO-Fu-NPs.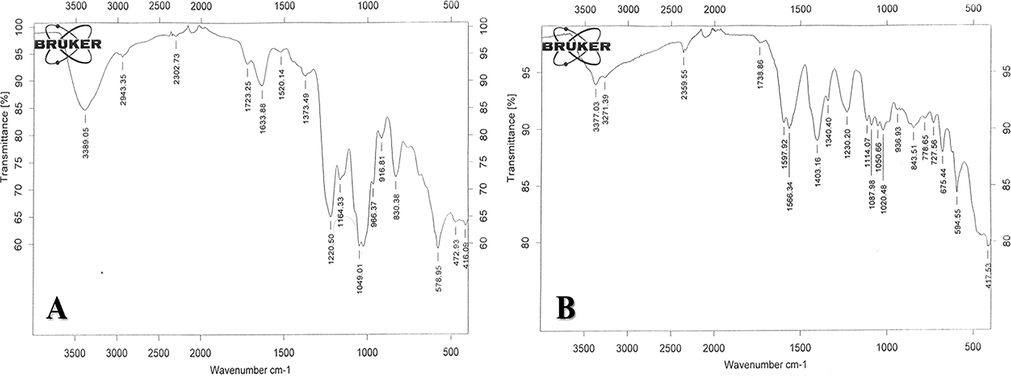
FTIR spectra of fucoidan (A) and synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) (B) in the wavelength range of 400–4000 cm−1.
The XRD pattern of the CuO-Fu-NPs was scanned within the 2θ range of 20–80° to determine the crystallographic planes of the fucoidan-capped nanomaterial. According to JCPDS Card No. 892531, the diffractions peaks observed at the 2θ values of 33.05°, 35.48°, 38.45°, 45.77°, 48.38°, 52.92°, 58.15°, 61.29°, 66.35°, and 67.57° (Fig. 3) are in covenant with the JCPDS Card no. 892531 and those are indexed were the (1 1 0), (0 0 2), (2 0 0), (1 1 2), (2 0 2), (0 2 0), (2 0 2), (1 1 3), (0 2 2), and (2 2 0) crystallographic planes, respectively (Suresh et al., 2016). Using the Scherrer equation, the <30 nm size of the NPs was calculated. Hence, it was concluded that fucoidan-capped CuO NPs had been successfully formed using the present methodology.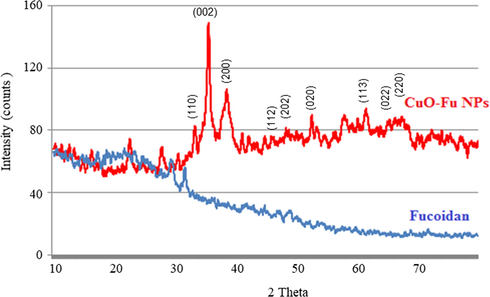
XRD spectral pattern of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs).
The morphology of the synthesized CuO-Fu-NPs was assessed using FE-SEM. The acquired images clearly displayed the polydispersed sphere morphology of the fucoidan-capped CuO NPs (Fig. 4A). The particle size of the CuO-Fu-NPs was due to the agglomeration of the capping agent (i.e., fucoidan) during NP formation, which changed their cluster form during the analysis. EDX analysis of the principal components of the prepared nanoproduct revealed peaks characteristic of CuO NPs at 1.00 keV and between 7.80 and 9.10 keV (Fig. 4B), which is in agreement with those previously reported (Biçer and Şişman, 2010)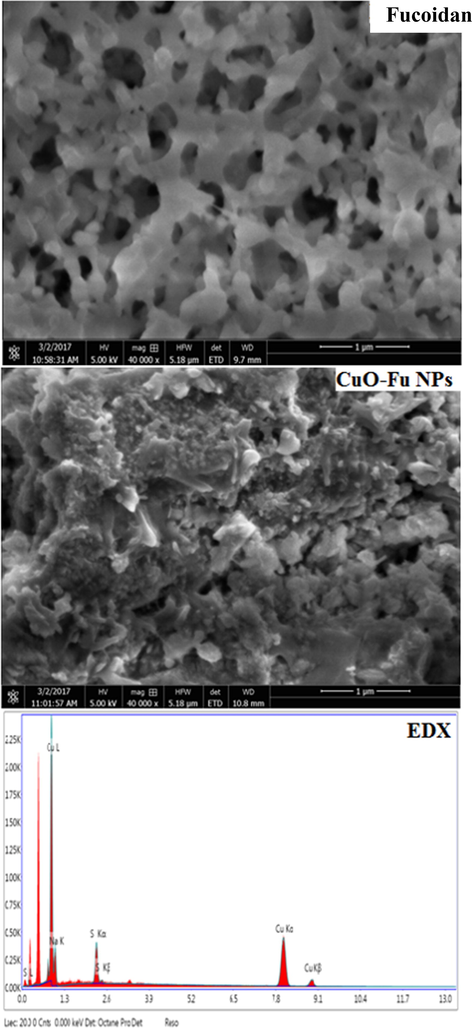
SEM image (A) and EDX spectrum (B) of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs).
3.2 Anticancer effect of the CuO-Fu-NPs
Cancer is prominent health issues these days, cervical cancer continues to constitute a major public health problem, about 311,000 deaths and 570,000 cases occurred in 2018. Approximately, 0.6 million cases of cervical cancer per year resulted fourth most common cancer in women (Arbyn et al., 2020). Copper compounds have been utilized for treatment of several ailments including cancer for thousands of years (Nagajyothi et al., 2017). Copper is a well-known metal, possessing anticancer effects. Furthermore, with the development in the field of nanotechnology, copper NPs can be used to target specific cancer cells with reduced adverse effects.
The effects of fucoidan alone and of the synthesized CuO-Fu-NPs on the proliferation of human cervical cancer (HeLa) cells were examined by MTT assay after treatment of the cells with increasing concentrations the test materials. Both fucoidan and the CuO-Fu-NPs respectively reduced the viability of HeLa cells in a dose- and time-dependent manner (Fig. 5A and 5B), indicating the anticancer potential of the synthesized nanoproducts. Previously we have also reported the anticancer effect of fucoidan against liver cancer cells (Phull et al., 2017a)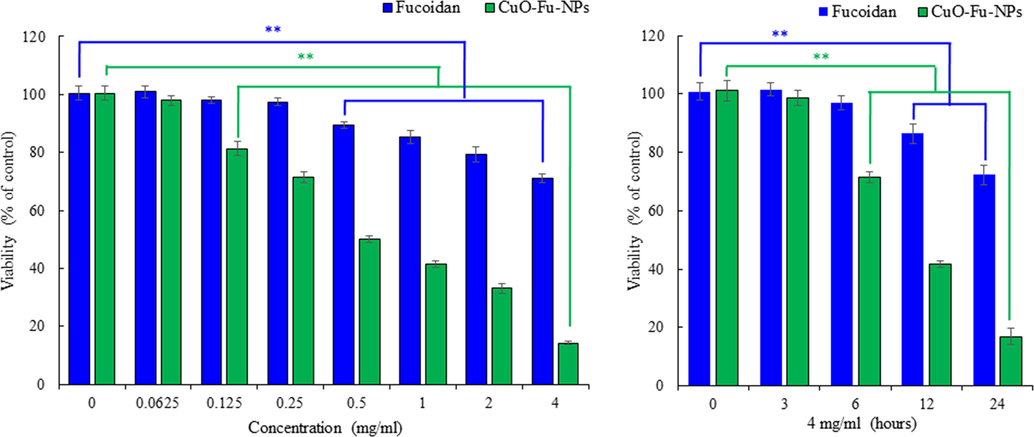
Effects of fucoidan alone and of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) on the viability of cervical cancer (HeLa) cells, as evaluated with the MTT assay. To determine the dose- and time-dependent effects on viability, cells were either treated with different concentrations of the test materials (i.e., 0–4 mg/mL) for 24 h (A) or with 4 mg/mL of the materials for different time periods (B). Untreated cells were considered as 100% viable. The experiment was repeated three times, and the results are shown as the mean ± SD. **p < 0.01, statistically significant difference compared with the non-treated cells.
The cytotoxic and pharmacological potential of the CuO-Fu-NPs and of fucoidan were assessed using anti-proliferation assays, whereupon their IC50 values were revealed to be 0.479 and 1.104 mg/mL, respectively. The lower IC50 value obtained with the CuO-Fu-NPs ultimately indicates the higher cytotoxic potential of the nanoproduct compared with that of fucoidan. These results were concordant with those obtained with CuO NPs prepared using Acalypha indica leaf extract, which showed significant toxic effects against human colon cancer (HCT-116) cells (Ganesan et al., 2020). Even though the copper compounds have strong therapeutic potential, our results agree with the results of Nagajyothi et al., 2017, who reported that anticancer effects of fucoidan associated CuO NPs on HeLa cells. The nanoparticles can suppress the viability of cancer cells through diverse mechanisms such as apoptosis, cell cycle inhibition.
The genotoxic effects of the test materials were assessed using the TUNEL assay, wherein TUNEL-positive cells indicate apoptosis-induced DNA strand breaks via the emission of a red fluorescence signal. Fig. 6 shows images of DAPI-stained nuclei (blue) and merged DAPI and TUNEL images. The number of red-stained nuclei was higher among the NP-treated cells than among the fucoidan-treated cells, indicating the amplified level of apoptosis induced by the CuO-Fu-NPs and, as such, their higher cytotoxic nature.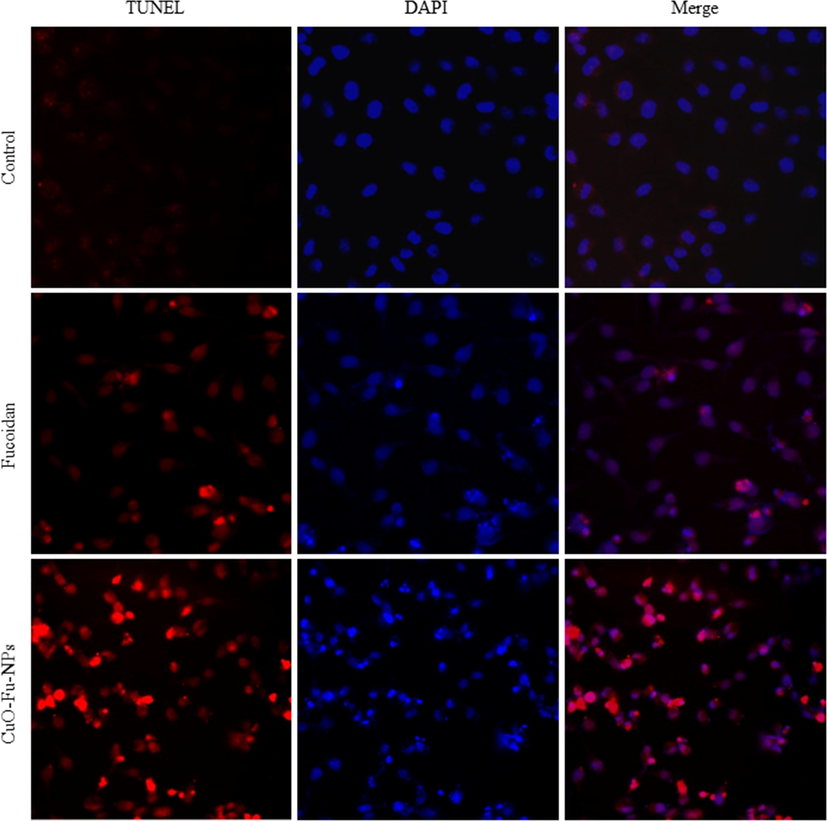
TUNEL assay of the cytotoxic and genotoxic effects of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) and of fucoidan alone. HeLa cells were exposed to 2 mg/mL of the test material for 24 h before assay.
3.3 Effect of CuO-Fu-NPs on apoptosis-related protein expression in cancer cells
Apoptosis controls number of cells and can be activated through both intrinsic and extrinsic pathways. A critical function for an anti-cancer compound is the activation of cancer cell apoptosis (Frankfurt and Krishan, 2003; Nagajyothi et al., 2017). It is essential process for keeping an equilibrium between cell death and division; avoidance of apoptosis results in unregulated cell proliferation, which can lead towards the diseases like cancer. Apoptosis is distinguished by morphological and biochemical modifications, and various cells in the same tissue do not undergo apoptosis at the same time.
Recent advancements in cancer treatments have focused on the discovery of novel anticancer by inducing apoptosis. In the novel apoptotic inducers or sensitizers have been used in conjunction with existing drugs against cancer. Defects in the apoptosis-inducing pathways can contribute to neoplastic cell proliferation (Jan and Chaudhry, 2019).
The caspases are important proteases that play vital roles in the activation and execution of cellular apoptosis. Therefore, the protein expression of caspase-3 and of its activated form (cleaved caspase-3) in the treated cancer cells was investigated. In the HeLa cells exposed to CuO-Fu-NPs, caspase-3 expression and activation were significantly induced (Fig. 7A, B). Various studies have shown the activation of caspase-3 and the caspase pathways during apoptosis induction in different cancer cells (Hou et al., 2020; Jiao et al., 2020; Xu et al., 2021). Furthermore, caspases play a critical role in apoptosis-associated DNA damage in cells (Jänicke et al., 1998; Kalaiarasi et al., 2018). The CuO-Fu-NPs also reduced the expression of the anti-apoptotic protein BCL2 and induced that of the pro-apoptotic protein BAX in the HeLa cells (Fig. 7C, D).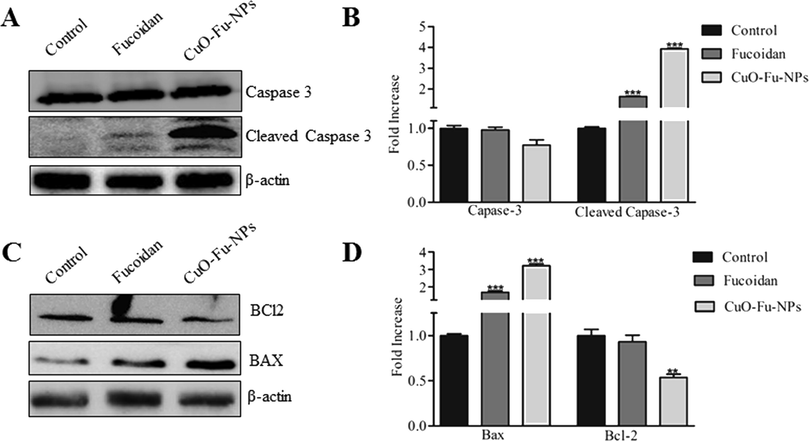
Effects of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) and of fucoidan alone on the protein expression of caspases, BCL2, and BAX. HeLa (cervical cancer) cells were untreated or treated with 2 mg/mL of the test material for 24 h, following which western blot using β-actin as the loading control (A, B). The experiment was repeated three times, and the data are presented as the representative results or the mean ± SD (C, D). The results were considered statistically significant at **p < 0.01 and ***p < 0.001.
The regulation of p53 protein expression by the test materials was also investigated. The CuO-Fu-NPs significantly induced p53 protein expression in HeLa cells relative to the level in non-treated cells (Fig. 8A, B). Such p53 activation activity further suggests the potential of these fucoidan-capped CuO NPs as anticancer agents (Kalaiarasi et al., 2018; Siddiqui et al., 2013). Moreover, HeLa cells exposed to CuO-Fu-NPs showed augmented expression of both cytochrome c and cleaved PARP (Fig. 8C). Given that cytochrome c initiates caspase-dependent apoptosis through activation of the caspase-3/PARP pathway (Kim et al., 2020), the results confirm that the CuO-Fu-NPs have apoptotic activity in HeLa cells.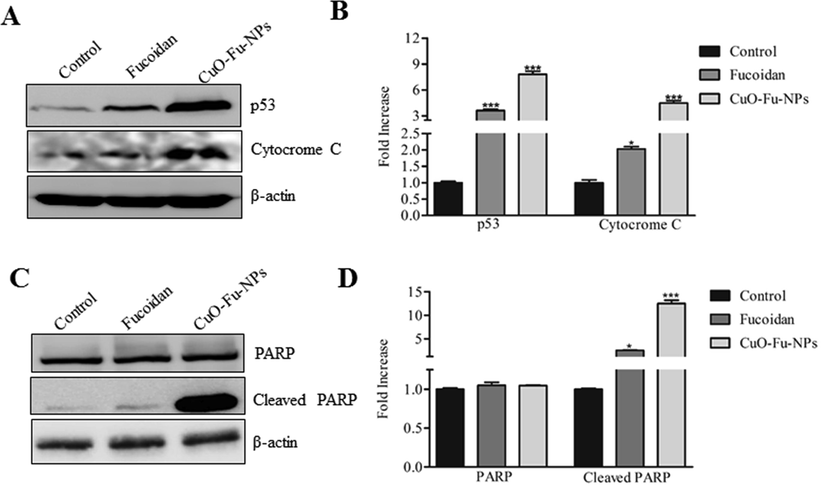
Effects of synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) and of fucoidan alone on the protein expression of p53, cytochrome c, and PARP. HeLa (cervical cancer) cells were untreated or treated with 2 mg/mL of the test material for 24 h, following which western blot assay of protein expression was carried out using β-actin as the loading control (A, B). The relative concentration of protein was determined using ImageJ densitometry software. The experiment was repeated three times, and the data are presented as the representative results or the mean ± SD (C, D). The results were considered statistically significant at **p < 0.01 or ***p < 0.001.
CuO NPs have been shown to execute their anticancer activity against a myelogenous leukemia (K562) cell line through the regulation of tumor suppressor genes and mitochondrial pathways (Kalaiarasi et al., 2018; Shafagh et al., 2015). The enhancement of the BAX/BCL2 ratio and the initiation of p53 serve to activate the release of cytochrome c from the mitochondria, stimulating the caspase cascade and apoptotic pathway (Siddiqui et al., 2013). Specifically, upon BCL2 protein activation by external compounds, BAX is inserted into the mitochondrial membrane and increases the membrane permeability, thereby releasing the cytochrome c molecules and consequently promoting apoptosis (Appaix et al., 2000; Kalaiarasi et al., 2018).
Previously, fucoidan-based nanoparticles have been tested to determine their potential as anticancer drug delivery for antitumor drugs, methotrexate, doxorubicin, cisplatin, curcumin and other drugs. Fucoidan from L. japonica and doxorubicin nanoparticles have been studied for anticancer therapy based pH-dependent release profile. It has ability to interact with P-selectin present in the cancer cells, resulted in the improved cell internalization and better inhibitory effects of designed nanoparticles than free DOX (Lu et al., 2017). In other in vivo study, fucoidan decorated doxorubicin nanoparticle showed that that intravenously injection increased anti proliferative and tumor inhibitory potential then doxorubicin alone. These augmented beneficial effects could be due to the immunomodulatory features of fucoidan, which were established in the in vitro investigations (Pawar et al., 2019). Furthermore, fucoidan and polyallylamine hydrochloride coated CuS nanoparticles exhibited higher anticancer activity then their components individually. In vitro experiments with human cervical cancer cells (HeLa) and human lung adenocarcinoma cells, as well as in vivo studies in mice injected with these cells (Jang et al., 2018). Binding capability with P-selectin is advantageous as intravenous delivery and therapeutic agent for detection of thrombosis. Like fucoidan coated poly(alkylcyanoacrylate) microcapsules have been administered intravenously in rats (Li et al., 2017).
Taking our results together, we postulate that the CuO-Fu-NPs synthesized in this study also induce anticancer and apoptotic effects through similar mechanisms to negatively affect the proliferation of HeLa cells (Fig. 9).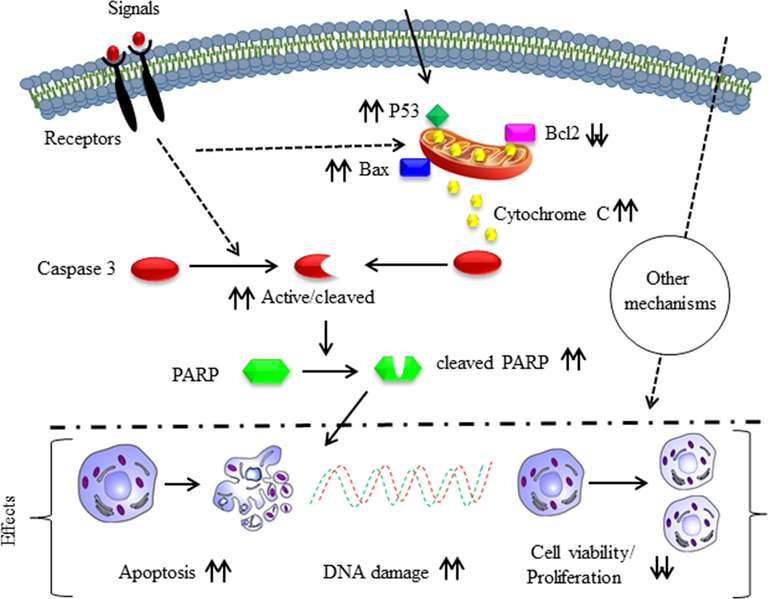
Anticancer mechanism of the synthesized fucoidan-capped copper oxide nanoparticles (CuO-Fu-NPs) in HeLa (cervical cancer) cells. The upward or downward double arrows respectively indicate increase in or the inhibition of the apoptosis-related protein expression and anticancer effects.
4 Conclusions
Nanobiotechnology signifies a new epoch and an innovative approach for the development and testing of new drug formulations based on NPs that have been produced using green methods or with natural substances as reducing agents. In this study, we demonstrated an eco-friendly green approach for the synthesis of CuO NPs with improved surface properties for the binding of biomolecules, using biocompatible polymer fucoidan. The synthesized CuO-Fu-NPs were characterized using spectroscopy, microscopy, and XRD techniques and their anticancer drug potential was demonstrated by investigating their toxicity toward HeLa cells. The CuO-Fu-NPs showed anti-proliferative and genotoxic effects on the cancer cells, and their ability to modulate apoptosis and growth signaling molecules was demonstrated using MTT, TUNEL, and western blot assays. The synthesized NPs were highly toxic toward HeLa cells, decreasing their viability with an IC50 value of 0.479 mg/mL compared with the 1.104 mg/mL value achieved with fucoidan alone. Their modulation of the apoptosis-related proteins BCL2, BAX, cytochrome c, activated caspase-3, and cleaved PARP led to DNA fragmentation and apoptosis in the cancer cells. Collectively, the results of this study indicate the significance of the use of naturally derived biocompounds for the expansion of organic and inorganic metallic NPs in natural product drug development having potential as anticancer drug carrier with along with its therapeutic effects. Further detailed research on the effects of CuO-Fu-NPs could translate into potential human health benefits, especially in cancer treatment.
Acknowledgement
The authors gratefully acknowledge the Korean government (MSIP) for supporting this study through National Research Foundation of Korea (NRF) funding (grant no: NRF-2019R1H1A2080051).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
All authors made substantial contributions to the study and have approved the manuscript for submission.
References
- Rapid spectrophotometric method for quantitation of cytochrome c release from isolated mitochondria or permeabilized cells revisited. BBA-Bioenergetics. 2000;1457(3):175-181.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng., C. 2019;102:536-540.
- [CrossRef] [Google Scholar]
- Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glo Health. 2020;8(2):e191-e203.
- [CrossRef] [Google Scholar]
- Controlled synthesis of copper nano/microstructures using ascorbic acid in aqueous CTAB solution. Powder Technol.. 2010;198(2):279-284.
- [CrossRef] [Google Scholar]
- Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif. Cells Nanomed. Biotechnol.. 2021;49(1):240-249.
- [CrossRef] [Google Scholar]
- Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs. 2016;14(3):42.
- [CrossRef] [Google Scholar]
- Nanoparticles in cellular drug delivery. Bioorg. Med. Chem.. 2009;17(8):2950-2962.
- [CrossRef] [Google Scholar]
- Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs. 2003;14(7):555-561.
- [CrossRef] [Google Scholar]
- Green synthesis of Copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab. J. Chem.. 2020;13(8):6802-6814.
- [CrossRef] [Google Scholar]
- Seaweed (Turbinaria ornata)-assisted green synthesis of magnesium hydroxide [Mg (OH) 2] nanomaterials and their anti-mycobacterial activity. Mater. Chem. Phys.. 2020;239:122007.
- [CrossRef] [Google Scholar]
- Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nut. Cancer. 2005;52(2):189-201.
- [CrossRef] [Google Scholar]
- Fruiting body polysaccharides of Hericium erinaceus induce apoptosis in human colorectal cancer cells via ROS generation mediating caspase-9-dependent signaling pathways. Food Funct.. 2020;11(7):6128-6138.
- [CrossRef] [Google Scholar]
- Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. bull.. 2019;9(2):205-218.
- [CrossRef] [Google Scholar]
- Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget. 2018;9(16):12649-12661.
- [Google Scholar]
- Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem.. 1998;273(25):15540-15545.
- [CrossRef] [Google Scholar]
- Nanoengineering in biomedicine: current development and future perspectives. Nanotechnol. Rev.. 2020;9(1):700-715.
- [CrossRef] [Google Scholar]
- Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J. Ethnopharmacol.. 2020;247:112256.
- [CrossRef] [Google Scholar]
- Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol let.. 2018;40(2):249-256.
- [CrossRef] [Google Scholar]
- Biosynthesis of Yttrium oxide nanoparticles using Acalypha indica leaf extract. Bull. Mater. Sci.. 2015;38(4):945-950.
- [CrossRef] [Google Scholar]
- Green and traditional synthesis of copper oxide nanoparticles—comparative study. Nanomaterials. 2020;10(12):2502.
- [CrossRef] [Google Scholar]
- Anticancer effect of mountain ginseng on human breast cancer: comparison with farm-cultivated ginseng. Evid-Based Compl. Altern. Med.. 2020;2020
- [CrossRef] [Google Scholar]
- Development of polymer microcapsules functionalized with fucoidan to target P-selectin overexpressed in cardiovascular diseases. Adv. Healthc. Mater.. 2017;6(4):1601200.
- [Google Scholar]
- The smart drug delivery system and its clinical potential. Theranostics. 2016;6(9):1306.
- [Google Scholar]
- Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym.. 2017;165:410-420.
- [CrossRef] [Google Scholar]
- Environmentally benign production of cupric oxide nanoparticles and various utilizations of their polymeric hybrids in different technologies. Coord. Chem. Rev.. 2020;419:213378.
- [CrossRef] [Google Scholar]
- Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem.. 2017;10(1):215-225.
- [CrossRef] [Google Scholar]
- Anticancer and antibacterial potential of Rhus punjabensis and CuO nanoparticles. Nat. Prod. Res.. 2020;34(5):720-725.
- [CrossRef] [Google Scholar]
- Introduction to Green Nanomaterials. In: Green Nanomaterials. Singapore: Springer; 2020. p. :1-21.
- [CrossRef] [Google Scholar]
- Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci.. 2011;76(3):T77-T83.
- [CrossRef] [Google Scholar]
- Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol.. 2019;122:1100-1114.
- [CrossRef] [Google Scholar]
- Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J. Pharm. Sci.. 2016;2(1):31-36.
- [CrossRef] [Google Scholar]
- In vitro antileishmanial, antibacterial, antifungal and anticancer activity of fucoidan from Undaria pinnatifida. Int. J. Biosci. 2017;11:219-227.
- [Google Scholar]
- Synthesis of silver nanoparticles using euphorbia wallichii extract and assessment of their bio-functionalities. Med. Chem.. 2020;16(4):495-506.
- [CrossRef] [Google Scholar]
- Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods. 2017;38:415-426.
- [CrossRef] [Google Scholar]
- Undaria pinnatifida a rich marine reservoir of nutritional and pharmacological potential: insights into growth signaling and apoptosis mechanisms in cancer. Nutr. Cancer. 2018;70(6):956-970.
- [CrossRef] [Google Scholar]
- In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol.. 2017;97:468-480.
- [CrossRef] [Google Scholar]
- Synthesis of a hierarchically structured Fe3O4-PEI nanocomposite for the highly sensitive electrochemical determination of bisphenol A in real samples. New J. Chem.. 2020;44(43):18633-18645.
- [CrossRef] [Google Scholar]
- Nanotechnology in biomedical applications: a review. Int. J. Nano Biomater.. 2010;3(2):119-139.
- [CrossRef] [Google Scholar]
- CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran. J. Basic Med. Sci.. 2015;18(10):993-1000.
- [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE. 2013;8(8):e69534.
- [CrossRef] [Google Scholar]
- FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci.: Adv. Mater. Devices. 2016;1(3):343-350.
- [CrossRef] [Google Scholar]
- Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym.. 2010;81(1):41-48.
- [CrossRef] [Google Scholar]
- Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol.. 2010;46(1):6-12.
- [CrossRef] [Google Scholar]
- Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem Toxicol.. 2021;111971
- [CrossRef] [Google Scholar]
- Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem.. 2009;57(18):8677-8682.
- [CrossRef] [Google Scholar]







