Translate this page into:
Synthesis of isosorbide bis(methyl carbonate) by transesterification of isosorbide with dimethyl carbonate, and evidence of its usefulness as a monomer for manufacturing polycarbonates
⁎Corresponding author. Tel.: (34) 671729328; fax: (34) 945198117. jramon.ochoa@tecnalia.com (José R. Ochoa-Gómez)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Isosorbide bis(methyl carbonate) (IBMC) is a barely explored isosorbide derivative potentially useful as a monomer for synthesizing biobased polymers. In this paper, its synthesis by transesterification of isosorbide (IS) with dimethyl carbonate (DMC) at reflux temperature using potassium and cesium carbonates as catalysts is reported. Using a semicontinuous operation mode, a 97% yield was obtained with cesium carbonate as catalyst at a concentration of 0.2 mol% vs. IS, and a DMC/IS molar ratio of 30. Oligomerization by reaction between IS, the intermediate isosorbide mono(methyl carbonate) and IBMC is a severe competitive reaction. Oligomers formation is strongly suppressed at high molar ratios and low catalyst concentrations. Cesium carbonate leads to a lower oligomer formation than potassium carbonate which is explained by the “cesium effect”. Evidence of the usefulness of IBMC as a biobased monomer for polymer synthesis through its polycondensation with 1,4-butanediol (1,4-BDO) is provided. A poly(isosorbide-1,4-BDO carbonate) with a weight average molar mass of 1800 g/mol and a polydispersity of 1.43 is obtained in 63.4% yield using 3 mol% DBU vs. IBMC as a catalyst with an IBMC/1,4-BDO molar ratio of 1 at 130 °C.
Keywords
Isosorbide
Isosorbide bis(methyl carbonate)
Biobased monomers
Transesterification
Biobased polymers
Cesium carbonate effect
1 Introduction
Moving from “chemicals from refineries” to “chemicals from biorefineries” is currently considered a must for sustainable development (European Commission, 2102, 2015). Isosorbide (IS) (1,4:3,6-dianhydro-d-glucitol) is an interesting example of an useful chemical produced in biorefineries since it is currently obtained by a double dehydration of sorbitol (Rose and Palkovits, 2012), which in turn is industrially synthesized by hydrogenation of glucose obtained from hydrolysis of polysaccharides such as starch and cellulose.
IS has a number of distinctive features making it a very attractive renewable raw material for manufacturing green materials able to compete advantageously with the current ones based on petroleum. Namely: (i) it could be broadly available at low cost in the near future when the methods for large scale manufacturing of glucose from non-food cellulose contained in lignocellulosics become fully industrialized; (ii) it is classified as a general recognized as safe (GRAS) material by the Food and Drug Administration; (iii) it is biodegradable; (iv) it bears two hydroxyl moieties of different reactivity, providing the possibility of designing a broad spectrum of derivatives with different properties and, therefore, applications; and (v) its rigid bicyclic structure provides improved mechanical strength to the polymers manufactured therefrom or derivatives thereof, for which reason, along with the above iv, IS has been proposed as an alternative to the toxic bisphenol A (Nelson and Long, 2012) in the manufacturing of epoxy resins and polycarbonates.
Consequently, it is not strange, on one hand, that it is already an industrial chemical platform for manufacturing polymers such as poly(ethylene-co-isosorbide)terephthalate (PEIT), polycarbonates, polyurethanes, polyesters and polymethacrylates, and chemicals such as isosorbide diesters and diethers, isosorbide di- and mononitrate, among others (Brack et al., 2011; El Boulifi et al., 2010; Fenouillot et al., 2010; Gallagher et al., 2015; Goerz and Ritter, 2013; Rose and Palkovits, 2012; Shirote and Bhatia, 2011; Van Haveren et al., 2007). And, on the other hand, that despite its current low production of about 31.5 kt/y in 2014 (http://www.marketresearchstore.com/report/isosorbide-market-z48041, 2016), the interest for IS and its derivatives is growing as shown by the recent news from Roquette, a world leader producer of IS, announcing the launch of the world’s largest IS production unit in Lestrem, France, with a capacity of 20,000 t/y (http://www.roquette.com/topic_id=11189/article_id=8959/, 2016).
Manufacturing of polycarbonates by bulk polycondensation of IS and dimethyl- or diethyl carbonate using potassium tert-butoxide, tin dioctanoate and titanium tetrabutoxide has been proved to be an unsuccessful method (Chatti et al., 2006). Instead, isosorbide alkyl ethers are obtained with these transesterification catalysts (Tundo et al., 2010; Aricò et al., 2014). An alternative could be the use of isosorbide bis(methyl carbonate) (IBMC, l,4:3,6-dianhydro-2,5-bis-O-(methoxy-carbonyl)-d-glucitol), an interesting and barely explored IS derivative belonging to the dianhydrohexitol bis(alkyl carbonate)s family. Its two linear carbonate end groups make IBMC a potentially useful monomer for the synthesis of biobased polymers such as the above mentioned polycarbonates and non-isocyanate polycarbonate-polyurethanes (by reacting with urethane-diols). In fact, Fuertes et al. (2013) reported the formation of a viscous polymer by reacting IBMC with ethylene glycol using cesium carbonate as a catalyst at stepwise growing temperatures between 115 and 165 °C while methanol is continuously distilled at vacuum. However, no data are given about its structure, molar mass and molar mass distribution.
A process for synthesizing dianhydrohexitol bis(alkyl carbonate)s useful to formulate ion-conductive electrolytes for batteries has been described by reaction between a dianhydrohexitol and a chloroformate ester (Abe et al., 2008), but the use of chloroformate esters is a major disadvantage due to their toxicity. A safer and environmental benign transesterification process (Scheme 1) avoiding toxic reactants has been claimed (Fuertes et al., 2013) in which IBMC is obtained in good yields by reacting IS with a large excess of dimethyl carbonate (DMC) in the presence of a transesterification catalyst, preferably K2CO3, LiOH and KOH, and shifting the equilibrium to the right by continuously distilling a mixture of methanol/DMC. Unfortunately, a high amount of oligomers is also formed, mainly at low DMC/IS molar ratios. Pure 99 wt% IBMC is obtained by a costly procedure involving the distillation of the solvent-free reaction mixture at 160 °C under high vacuum (<1 mbar) on a wiped-film evaporator in short-path configuration. However, no systematic study of the reaction is reported. Some additional but also few data of this reaction have been reported by Aricò et al. (2014).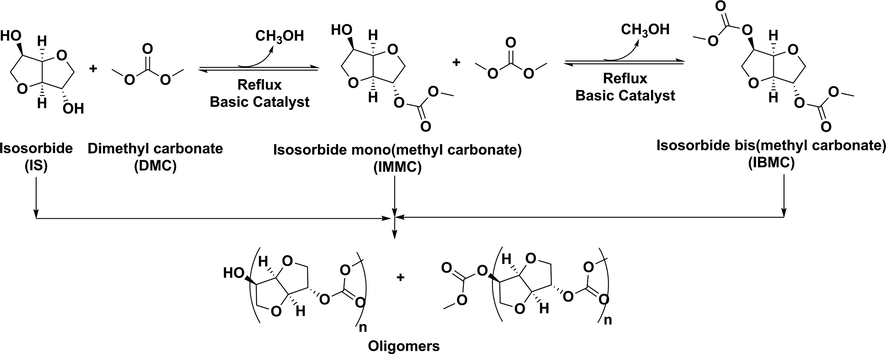
Synthesis of isosorbide bis(methyl carbonate) and oligomers thereof. Only the 2-exo isomer of IMMC is shown.
In this paper, we report the synthesis of IBMC from IS and DMC by transesterification at reflux temperature using potassium and cesium carbonates as catalysts, studying the influence of operation mode, catalyst nature, catalyst concentration and reactants molar ratio on both IBMC and oligomer yields with special emphasis on looking for suitable conditions for preventing or minimizing the oligomer formation to avoid or simplify a purification procedure. Likewise, evidence about the usefulness of IBMC for obtaining polycarbonates by polycondensation with 1,4-butanediol (1,4-BDO) is provided.
2 Experimental
2.1 Materials
All chemicals were of analytical grade and were purchased from Sigma-Aldrich (Madrid, Spain).
2.2 General reaction procedure for IBMC synthesis
In the semicontinuous mode, IS (10 g, 0.0674 mol) and the DMC required amount to attain the desired molar ratio were introduced into a 100 mL glass reactor fitted with a magnetic stirrer, a thermometer and a reflux condenser. The reaction mixture was heated at the reflux temperature in a glycerol bath kept at 100 °C, and then the needed amount of catalyst to reach the desired catalyst/IS molar ratio was added (all catalyst concentrations are given in mol% vs. IS). The reaction was kept under stirring for 0.5 h, with temperature of the reaction mixture decreasing continuously indicating methanol formation. Then, the reflux condenser was replaced by 18 theoretical plates Vigreux rectifying column connected to a condenser so hereinafter the reaction proceeded by continuously removing methanol by distilling a mixture of methanol and DMC until the reaction mixture temperature reached that corresponding to the boiling point of DMC (90 °C). The heat flow was adjusted so that the temperature of the vapors at the top of the column was always below 75 °C in order to avoid an excessive loss of DMC. DMC losses by distillation were continuously determined by measuring the distillate density, and replaced by adding fresh DMC in order to keep the initial molar ratio constant. Reaction was completed when no hydroxyl band was observed by FTIR (Fig. 1) and methanol was no longer detected in the distillate as shown by density measurements. Then, the reaction mixture was cooled at room temperature and the catalyst was removed by filtration (recovery >99% for K2CO3, and variable for Cs2CO3 depending on its concentration because it is partially soluble in reaction medium), the cake was washed with DMC and the combined filtrates were evaporated under vacuum to yield a slightly yellow residue which crystallized on standing into a waxy solid when it was pure IBMC, but remained as a viscous liquid if the content of oligomers is high enough. IBMC was identified by 1H NMR, 13C NMR, elemental analysis and GC-MS, when a high DMC/IS molar ratio of 30 and a low 0.2 mol% concentration of cesium carbonate were used. In the batchwise mode, the reaction was carried out by mixing all reactants, heating to reflux temperature, then adding the catalyst and monitoring the reaction progress by FTIR. Reaction was over when the ratio between the absorbance of the hydroxyl band (∼3450 cm−1) and that of the carbonyl group of IBMC (1751 cm−1) was constant over time. NMR spectra are given in Figs. S1–S6.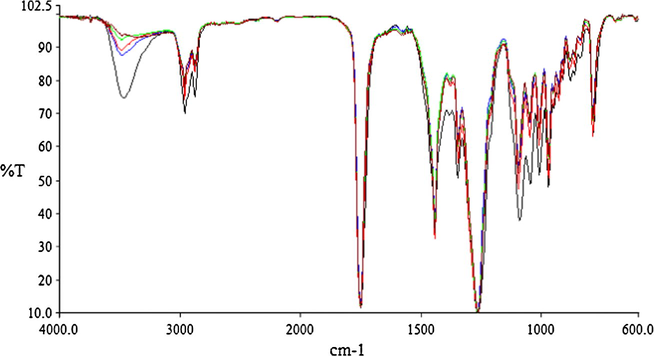
FTIR monitoring of isosorbide bis(methyl carbonate) synthesis in the semicontinuous mode. DMC/isosorbide molar ratio: 10. Catalyst: K2CO3, 2 mol% vs. isosorbide. Black: 1 h, Blue: 1.42 h, Red: 1.83 h, Green: 2.16 h, Tan: 3 h.
IBMC, FTIR νmax/cm−1 (film): 2961, 2880, 1749, 1444, 1349, 1263, 1100, 1053, 1011, 973, 933, 887, 867, 791. 1H NMR (500 MHz, DMSO-d6; Me4Si) δ (ppm) 5.04 (q, J = 4.9 Hz, 1H), 4.97 (d, J = 3.3 Hz, 1H), 4.81 (t, J = 5.3 Hz, 1H), 4.46 (d, J = 5.0 Hz, 1H), 3.96 (d, J = 10.9 Hz, 1H), 3.84–3.76 (m, 3H), 3.73 (s, 3H), 3.72 (s, 3H). 13C NMR (126 MHz, DMSO-d6; Me4Si) δ (ppm) 155.03 (C⚌O), 154.79 (C⚌O), 85.76 (CH—O), 81.22 (CH—O), 81.09 (CH—O), 77.20 (CH—O), 72.79 (CH2—O), 70.81 (CH2—O), 55.46 (OCH3), 55.38 (OCH3). Elemental analysis: Found (%): C, 45.94; H, 5.36. Calc. (%) for C10H14O8: C, 45.81; H, 5.38. MS (m/z): 41, 43, 45, 55, 59, 68, 69, 71, 77, 81, 84, 85, 99, 103, 110, 129, 131, 231, 263.
2.3 Polycondensations
Polycondensations of IBMC (typically between 2 and 5 g) and 1,4-BDO were carried out by mixing both chemicals at the desired molar ratio, heating up to the chosen reaction temperature under stirring, adding the catalyst (3 mol% vs. IBMC), and monitoring the polymerization by ATR-FTIR until the absorbance of the OH band at 3350 cm−1 was constant.
Polycondensation of dimethyl carbonate (DMC) and 1,4-BDO was carried out in a 500 mL jacketed reactor under mechanical stirring. 1,4-BDO (2.2 mol) and DMC (2,2 mol) were mixed and heated at 90 °C. Then, Cs2CO3 (0.022 mol) was added as a catalyst and reaction was carried out by distilling continuously a mixture of DMC and methanol. DMC losses as determined by measuring the distillate density were replaced by adding continuously the same amount of DMC. Reaction was stopped when no more MeOH was distilled.
2.4 Analytical methods
IS and IBMC amounts in IBMC synthesis were analyzed by HPLC using a Varian apparatus Model 920-LC, fitted with both a refractive index detector Varian 920-LC and a reverse phase Synergi C12 MAX-RP column, 4 μm, 150 mm × 4.6 mm. Samples for analysis were prepared by diluting aliquots of the isolated product (after catalyst removal from the reaction mixture by filtration and vacuum solvent evaporation of the filtrate) in the mobile phase (55:45 v/v acetonitrile:acetic acid). Flow rate was 1.0 mL/min, and column and detector temperatures were 35 °C and 40 °C, respectively. Calibration lines using IS and IBMC concentrations between 50 and 1000 ppm were carried out with no internal standard. IS and IBMC retention times were 1.35 min and 2.3 min, respectively.
IS and 1,4-BDO amounts after polymerizations of IMBC with 1,4-BDO were determined in the same HPLC apparatus and detector but with an Aminex HPX-87 column in a 0,01 N H2SO4 mobile phase at a 0.7 mL/min flow rate. Column and detector temperatures were 65 °C and 50 °C, respectively. Calibration lines using IS and 1,4-BDO concentrations between 50 and 1000 ppm were carried out using oxalic acid (185 ppm) as an internal standard. Oxalic acid, IS and 1,4-BDO retention times were 5.66, 10.92 and 17.22 min, respectively.
Hydroxyl values of reaction samples, after catalyst separation by filtration and DMC/methanol removal by evaporation, were determined according to ISO 14900:2001.
Contents of oligomers were determined by gel permeation chromatography (GPC) on an area basis using a Polymer Laboratories equipment model PL-GPC-50 Plus fitted with a refractive index detector. Eluent was tetrahydrofuran and polystyrene standards from Easy Cal PS-2 were used to obtain a molar mass versus elution time calibration line with a 300 × 7.5 mm Resipore column at 40 °C. Injection volume was 20 μL, flow rate 1 mL/min and the concentration of samples was as high as 10,000 ppm in THF for detecting unambiguously all oligomer species. Fuertes et al. (2013) calculated the contents of oligomers by Gas Chromatography based on area percentages relative to the total area of all detected peaks, with the solvent being excluded. This method seriously underrates the percentage of oligomers because those of higher polymerization degree are not detected due to their high boiling points, as shown by Ochoa-Gómez et al. (2015).
Molar masses and polydispersities of polymers obtained by polycondensation of IMBC and 1,4-BDO as well as dimethyl carbonate and 1,4-BDO were obtained with the same equipment and polystyrene standards using 0.1 wt% LiBr in dry DMF as eluent at 1.0 mL/min with a 300 × 7.5 mm Polar Gel-L column at 50 °C. Injection volume was 20 μL and concentration of samples was 5000 ppm in DMF.
IBMC and isosorbide mono(methyl carbonate) (IMMC) were identified by GC-MS using a Gas Chromatograph Agilent GC 6890, a MS detector 5973 and a HP5-ms column of 30 m, 0.25 mm and 0.25 μm. Analytical conditions: samples dissolved in acetonitrile; injector temperature, 225 °C; Split, 1:50; injection volume, 1 μL; oven temperature started at 40 °C (3 min) and was increased at 7 °C/min up to 300 °C and kept at this temperature for 10 min; injection mode: Scan; helium at 1 mL/min was used as the carrier gas; mass range: 33.0–550.0; running time, 50.14 min. Retention times: IS, 16.80 min; IBMC, 25.24 min; IMMC, two peaks at 20.40 y 21.87 min, both with the same MS spectrum, and corresponding to the two 2-exo and 5-endo isomers. The peak at 20.40 min was always in a higher proportion than that at 21.87 min. Aricò et al. (2014) studied this same reaction in a batchwise mode under reflux for 6 h with a DMC/IS molar ratio of 30, using K2CO3 as a catalyst in amounts ranging from 10 to 100% mol% vs. IS, and isolated one of the IMMC isomers by column chromatography. Its structure was identified by 1H NMR and resulted to be the exo isomer. They also found that the exo isomer was always present in the reaction mixture in a greater amount than the endo one, as the exo-hydroxyl group in IS is much more reactive because the endo isomer is forming an intramolecular hydrogen bond with the furanic oxygen in beta position. Consequently, we can assume that the peak at 20.40 min corresponds to the 2-exo isomer, the one shown in Scheme 1, while that at 21.87 min corresponds to the 5-endo isomer. From Table 2 of the above reference it can be deduced that the exo/endo ratio ranges between 2 and 4 depending on experimental conditions, with the ratio increasing as catalyst concentration decreases. Based on GC-MS results we found a 20.40 min/21.87 min area ratio = 2 for a reaction carried out in a batchwise mode for 6.5 h under reflux, at a DMC/IS molar ratio of 5 using K2CO3 as a catalyst in an amount of 10 mol% vs. IS.
Infrared spectra of samples were obtained after solvent evaporation in both a FTIR Perkin Elmer Spectrum 2000 spectrometer and a Bruker ALPHA Platinum-ATR-FTIR Spectrometer.
3 Results and discussion
3.1 Operation mode
As depicted in Scheme 1, reaction between IS and DMC is ruled by equilibrium. Therefore, continuous removal of methanol (semicontinuous operation mode) is imperative for shifting the equilibrium to the right and achieving a 100% IS conversion. If a batchwise procedure is used, reaction never becomes complete regardless of operating conditions. Even at a DMC/IS molar ratio so high as 20, the ratio between the absorbance of the hydroxyl band (∼3450 cm−1) and that of the carbonyl group of IBMC (1751 cm−1), which should be zero at a 100% IS conversion, remains constant between 0.15 and 0.28 after 0.5–3 h depending on reaction conditions (Fig. 2), as determined by FTIR measurements. Using 10 mol% K2CO3 as a catalyst, IS conversions were ranged between 84.5% and 90% at 5–20 DMC/IS molar ratios, and hydroxyl values of isolated product were comprised between 196.2 and 235.7 mg-KOH/g, also indicating incomplete reaction. These results agree with those obtained by Aricò et al. (2014) who reported also an incomplete reaction between IS and DMC in a batchwise procedure with a DMC/IS molar ratio of 30 in the presence of a stoichiometric amount of K2CO3 (1 mol. eq.), under reflux at 90 °C. After 6 h, IS conversion was 100% but IBMC selectivity was only 54%. The intermediate exo- and endo-monocarboxymethyl derivatives were obtained in 37% and 9% yields, respectively. The use of dried K2CO3 in a lower amount (0.1 mol. eq.) resulted in an increase of IBMC yield up to 90%, but exo- and endo-monocarboxymethyl intermediates were still present in the reaction mixture in 8% and 2% yields, respectively.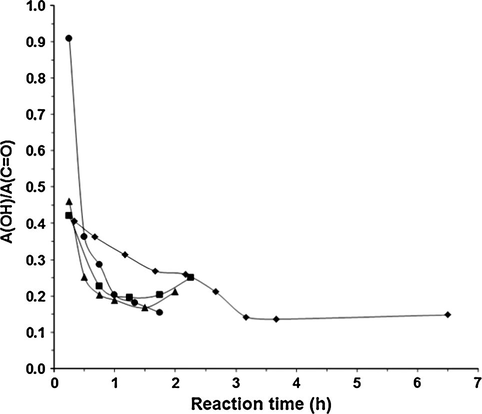
FTIR monitoring of the batchwise isosorbide bis(methyl carbonate) synthesis at reflux temperature. Catalyst: K2CO3, 10 mol% vs. isosorbide. DMC/isosorbide molar ratios: (●) 20; (▴) 15; (■) 10; (♦) 5.
On the other hand, polycondensation of IS, IMMC and IBMC leading to polycarbonates could occur (Scheme 1). This was confirmed by GPC of batchwise reactions in which a great amount of low molar mass oligomers with a maximum polymerization degree of 3–4 were formed instead of polymers.
3.2 Influence of catalyst nature and concentration
The influence of catalyst concentration on yields of IBMC and oligomers is depicted in Fig. 3 at a DMC/IS molar ratio of 10 for the semicontinuous process and for two catalysts: K2CO3 and Cs2CO3. As it can be seen, oligomers formation increases sharply with catalyst concentration for both catalysts from 1 to 10 mol%, and then more slowly up to 150 mol%, reaching values as high as 20% for K2CO3. Conversely, the lower the catalyst concentration, the higher the IBMC yield. Same result was found by Aricò et al. (2010) who reported an increase in IBMC yield from 85% to 90% when K2CO3 concentration was decreased from 100 mol% to 10 mol% vs. IS, working in batch for 6 h under reflux at a DMC/IS molar ratio of 30.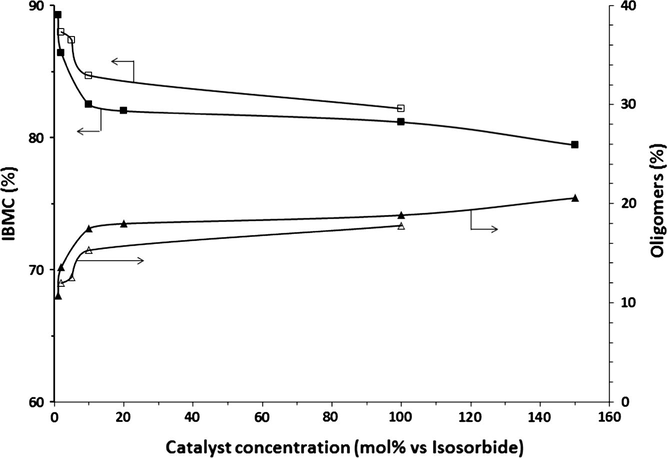
Variation of IBMC (■) and oligomers yields (▴) as a function of the catalyst concentration at a DMC/IS molar ratio of 10. K2CO3: filled symbols; Cs2CO3: unfilled symbols.
Due to the large DMC excess (5-fold the stoichiometric) used, oligomers with carboxymethyl end groups are mainly expected, although hydroxy ended oligomers could also be formed as depicted in Scheme 1. This possibility was checked by analyzing the hydroxyl values of the isolated products obtained using K2CO3 as a catalyst. Hydroxyl values ranged between 4.7 and 19.2 mg-KOH/g despite IS conversions were 100% in all cases and no IMMC was detected, indicating the presence of a small fraction of hydroxy ended oligomers.
The strong influence of catalyst concentration on oligomers formation can be explained by assuming that the reaction rate for monomer (IBMC) synthesis is much higher than that for oligomerization. Consequently, a decrease in catalyst concentration leads to a decrease in both reaction rates but the rate of oligomerization can decrease below a threshold value leading to a higher monomer/oligomers ratio at low catalyst concentration than at high catalyst one.
On the other hand, oligomer contents were lower for cesium carbonate than for potassium carbonate. This seems another example of the well-known “cesium effect” (Galli, 1992) a term used to describe the advantages concerning better yields and milder reaction conditions of cesium catalyzed reactions as compared to non-cesium catalysts. The large cationic radius, low charge density and large polarizability make the cesium ion to stand out among the other alkali metal ions. Thus, cesium carbonate is the stronger base of the alkali metal carbonates family and its solubility in organic solvents, such as N,N-dimethylformamide, dimethyl sulfoxide and sulfolane, is much higher than that of potassium carbonate (Cella and Bacon, 1984). We have measured the solubility of cesium carbonate in DMC and 10 wt% MeOH in DMC at room temperature obtaining values of 0.67 and 6.71 g/L, respectively, while solubilities of potassium carbonate in the same solvents are negligible. Consequently, the observed lower oligomer contents when cesium carbonate catalyzes the reaction herein reported can be explained due to a double effect. The first one is its higher base strength, thereby speeding up the reaction rate for IBMC synthesis in a higher extent than that for oligomers formation. The second one is that while the reaction proceeds under heterogeneous catalysis for potassium carbonate, a homogeneous catalysis at low catalyst concentration or a mixed homogeneous-heterogeneous catalysis at high catalyst concentration occurs for cesium carbonate under the experimental conditions studied. The homogenous catalysis speeds further up the reaction rate for IBMC synthesis contributing to decrease the oligomer contents.
The influence of Cs2CO3 concentration for the semicontinuous process in the 0.2–40 mol% range on yields of IBMC and oligomers but at a DMC/IS molar ratio of 30 instead of 10 is depicted in Fig. 4. As in the case of potassium carbonate, oligomers formation decreases as cesium carbonate concentration decreases, but in this case the higher DMC/molar ratio favors an additional decrease in oligomer concentration, reaching a value as low as 3% at a 0.2 mol% cesium carbonate concentration. The purity of IMBC (97%) at this concentration is high enough for avoiding a costly and cumbersome purification procedure. This small amount of oligomers must not necessarily be separated from the isolated IMBC because in some applications, such as in the synthesis of polymers, they can work equivalently to IMBC as it has been reported (Fuertes et al., 2013).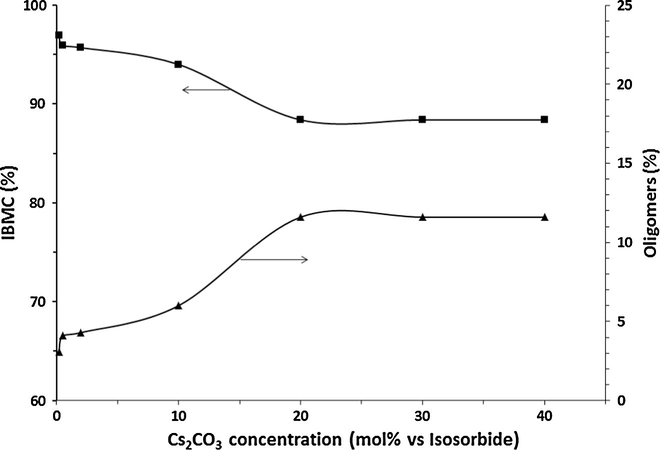
Variation of IBMC (■) and oligomers yields (▴) as a function of the Cs2CO3 concentration at a DMC/IS molar ratio of 30.
On the other hand, unlike potassium carbonate, cesium carbonate is active even at a concentration as low as 0.2 mol%, which can be attributed to its stronger basic character as well as to its total (at low concentration) or partial (at high concentration) solubility in the reaction medium leading to a homogeneous catalysis.
3.3 Influence of isosorbide/dimethyl carbonate molar ratio
The influence of DMC/IS molar ratio on yields of IBMC and oligomers is depicted in Fig. 5 at a catalyst (K2CO3) concentration of 2 mol% vs. IS. According to results reported in Section 3.2, cesium carbonate would be the catalyst to be chosen. However, potassium carbonate was ultimately selected because it is much cheaper than cesium carbonate, which is a key point facet from an industrial standpoint.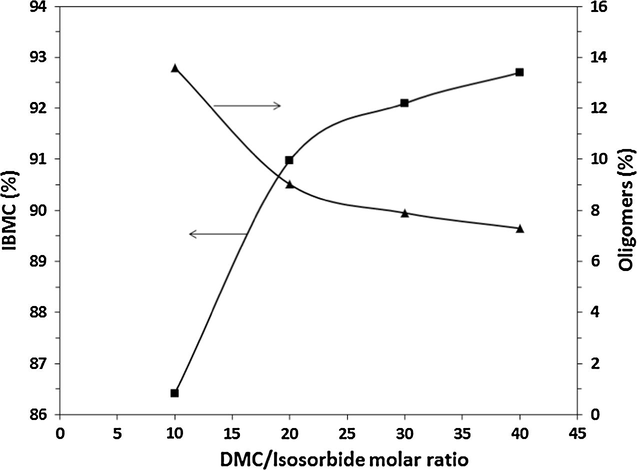
Variation of IBMC (■) and oligomers yields (▴) as a function of the DMC/isosorbide molar ratio. Catalyst: K2CO3, 2 mol% vs. isosorbide.
As it can be seen, IBMC yield increases with DMC/IS molar ratio leading to a high 93% IBMC yield at a molar ratio of 40, while oligomers yields decrease with DMC/IS molar ratio from 13.6% at 10 to 7.3% at 40. On one hand, these results can be explained taking into account that an increase in molar ratio shifts further equilibrium to right, thereby increasing reaction rate for IBMC formation. As oligomerization is a consequence of the reaction between hydroxyl moieties of IS and IMMC and methoxycarbonyl groups of IMMC and IBMC, an increase in reaction rate for IBMC formation decreases half-life of hydroxyl groups in reaction media leading to a lower oligomer/IBMC ratio. On the other hand, the higher the DMC/IS molar ratio the lower the concentration of synthesized IMBC as well as of IS and intermediate IMMC, leading to a lower probability of oligomerization.
3.4 Polycondensation of IBMC and 1,4-BDO
Polycondensation between IBMC and 1,4-BDO was explored to assess the capability of IBMC to be used as a monomer in the synthesis of isosorbide derived polycarbonates. The aim of this preliminary study was to show that this possibility exists and is high, but it is not to develop a fully optimized polycondensation process with a polymer yield close to 100% and under conditions leading to a wide range of tunable molar masses. This last objective requires further experimental work and it is out of the scope of this paper.
Reaction conditions, conversions of IBMC and 1,4-BDO, yields of IS (YIS) and polymer (Yp), and normalized absorbances of OH bands at ∼3450 cm−1 (AOH) are given in Table 1, in which nature of the base, temperature, pressure, reaction time and operation mode (batchwise and semicontinuous) were studied. Yp corresponds to the percentage area obtained by GPC (see Table 2) for peak 4, the only one which can be assigned to a polymer because peak 1 corresponds to IS and peaks 2 and 3 have both a polydispersity of ca. 1 and a low molar mass of about 216 and 338, respectively, indicating that they are not polymers. Molar ratio IBMC:1,4-BDO = 1; Catalyst concentration: 3 mol% vs. IBMC. C: conversion; YIS: yield of isosorbide; Yp: Yield of polymer (peak 4 in Table 2).
Run
Catalyst
T (°C)
P (bar)
tr (h)
CIBMC (%)
C1,4-BDO (%)
YIS (%)
Yp (%)
AOH (cm−1)c
1a
Cs2CO3
160
0.6
2
100
100
43.2
47.8
0.257
2a
DBU
130
1.0
4
100
100
26.2
63.4
0.098
3a
DBU
160
0.6
2
100
100
34.0
61.1
0.116
4b
DBU
130
1.0
4
100
100
35.9
52.8
0.158
Run
Peak #
Relative areas (%)/Mw/Polydispersity
1 (IS)
2
3
4
1
24.7/98/1.02
13.5/215/1.02
14.1/338/1.01
47.8/946/1.28
2
17.6/100/1.02
9.1/216/1.02
9.9/335/1.01
63.4/1243/1.43
3
16.4/100/1.03
11.6/219/1.02
10.9/338/1.01
61.1/1142/1.40
4
25.8/103/1.03
10.7/220/1.02
10.7/343/1.01
52.8/1114/1.36
At first, two reaction routes are possible as depicted in Scheme 2. Route I leads to IS and 1,4-BDO mono- and di-carbonates, which in turn could react with other 1,4-BDO molecules yielding poly(1,4-butanediol carbonates), and Route II resulting in poly(isosorbide-co-1,4-BDO carbonate). Isosorbide is formed in yields ranging from ca. 26% to ca. 43% as determined by HPLC, being an evidence of the Route I existence. The IS levels are in accordance with AOH values of polymerization samples as obtained by ATR-FTIR, with IS levels increasing with AOH values, indicating that hydroxyl values are mainly derived from the existence of free IS in the reaction mixture because 1,4-BDO conversions were 100% in all cases. Likewise, there are some evidences of the Route II existence. On one hand, both IBMC and 1,4-BDO conversions were 100% in all cases, and, on the other hand, IS was not fully removed from IBMC via Route I evidenced by the fact that IS yields are always below 50%. Consequently, a condensation between IBMC and 1,4-BDO leading presumably to poly(isosorbide-co-1,4-BDO carbonate) has occurred as discussed later.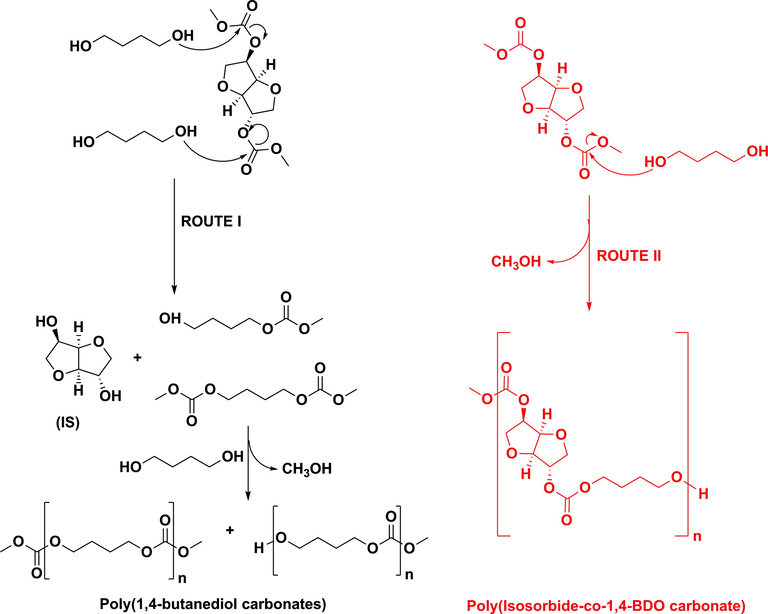
Polycondensation routes of IBMC and 1,4-BDO under basic catalysis.
The undesired route I leading to IS strongly depends on catalyst nature being less active with the amidine-type catalyst DBU (1,8-Diazabicyclo[5.4.0]undec-7-ene) than with cesium carbonate (compare runs 1 and 3). It is difficult to conclude whether this effect is due to a difference in basicity between both bases or to a differential steric effect as DBU has a more bulky structure than cesium carbonate. Reported base strengths of DBU and cesium carbonate in different solvents (Steinmetz, 2011) are dependent of solvent nature. Thus, they are similar in acetonitrile and N-methyl-2-pyrrolidone, but differ in dimethyl sulfoxide and dimethylformamide (in both cases cesium carbonate basicity is higher than that of DBU), and in 1,2-dimethoxyethane (DBU basicity is much stronger than that of cesium carbonate). Consequently, a further study for determining the basicity of both catalysts under the polymerization conditions used would be useful to ascertain the relative weight of catalyst basicity and DBU steric effect is needed.
Working under vacuum speeds up the reaction but apparently has no influence on relative weight of both conversion routes. However, temperature seems to have an important influence with lower temperatures favoring IBMC-1,4-BDO polymer formation (compare runs 2 and 3). A possible cause will be discussed later. The semicontinuous operation mode has a negative influence on route II (compare runs 2 and 4), although the main species formed is also a polymer, with this expectedly being poly(isosorbide-co-1,4-BDO carbonate) as shown below. Anyway, these results show the great importance of catalyst and temperature on relative weight of both conversion routes and open the way for finding a catalyst or catalytic system together with some polymerization conditions leading predominantly to polymer formation.
Weight average molar masses (Mw) together with relative areas (%) of peaks detected by GPC in 0.1 wt% LiBr in DMF are shown in Table 2.
As it can be seen, the major component in the polymerization mixture is a polymer (peak 4) of a low molar mass ranging between 946 and 1243 and a polydispersity of about 1.40, affording for 47.8–63.4% of the reaction mixture depending on reaction conditions. Peak 1 corresponds to IS as checked by injecting pure IS. Peaks 2 and 3 have a polydispersity of ca. 1 and low Mw between 215 and 343, indicating that they are not oligomers but most probably chemicals resulting from the condensation of 1 molecule of IBMC and 1 or 2 molecules of 1,4-BDO, and/or derivatives thereof. As these chemicals have hydroxy or hydroxy/carboxymethyl terminal groups, the question arising is why their polycondensation has not proceeded. A possible explanation is depicted in Scheme 3 for an exemplary molecule (isosorbide (4-hydroxybutyl carbonate) (methyl carbonate), I) resulting from the condensation of 1 molecule of IBMC and 1 molecule of 1,4-BDO. I can undergo a number of reactions in the presence of 1,4-BDO, IBMC and IS. The transesterification with IS, 1,4-BDO or IBMC (not depicted in Scheme 3) would lead to a molecule of higher molar mass which after further transesterification would lead to a polycarbonate, as desired. However, there are other reactions preventing or slowing down further polycondensation. One of them is an intramolecular transesterification resulting in the cyclic molecule II, which is favored by the V-shape of the two IBMC furanic rings allowing the hydroxyl moiety in I to be in the vicinity of the oxo group in the carboxymethyl moiety of the same molecule. II can polymerize by ring-opening provided that a diol or polyol is present in the reaction medium, but its formation slows down the desired polycondensation reaction and consumes 1,4-BDO preventing the molar mass increase of the desired polymer. No peak of cyclic carbonate was detected by FTIR (1780–1790 cm−1). Therefore, II is not formed or, if formed, it reacts very quickly with 1,4-BDO and/or IS leading to a linear structure by ring-opening. The other reaction is the methylation of the hydroxyl moiety leading to compound III (isosorbide (4-methoxybutyl carbonate) (methyl carbonate)), with IBMC being the methylating agent. III cannot longer undergo polycondensation. As far as we know, there is no example in the literature showing the use of IBMC as a methylating agent, unlike DMC. Thus, at 90 °C (reflux temperature of DMC), DMC behaves as an ester, and a methoxycarbonylation reaction takes place through a BAc2 mechanism, while at temperatures higher than 120 °C it behaves as a methylating agent via a BAl2 mechanism (Tundo, 2001; Tundo et al., 2010). Therefore, at the temperatures used in the polycondensation (130–160 °C) the behavior of IBMC as a methylating agent must not be discarded, at least in a certain extent. Molar masses of II and III are consistent with those obtained by GPC for peaks 2 and 3. On the other hand, this could also explain the abovementioned decrease in total yield of peaks 2 and 3 as temperature decreases (runs 2 and 3 in Table 2) because a decrease in the methylation degree is expected when temperature decreases.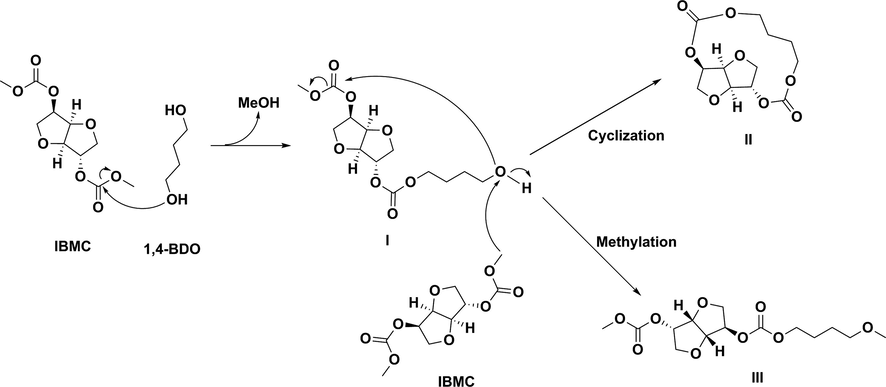
Reactions justifying formation of some possible chemicals related to peaks 2 and 3 in GPC.
As it can be deduced from data in Table 2, there is also a correlation between molar masses of Peak 4 and IS yields obtained (Table 1), the lower the IS yield the higher the molar mass, as expected. With the polystyrene standards used, the molar mass obtained by GPC for IS is 100 ±2.5 on average, i.e. 1.46 times lower than its actual molar mass. Assuming that the hydrodynamic volume of all species incorporating IS is similar we can consider that molar mass for polymers corresponding to peak 4 is 1.46 times higher than that reported in Table 2. Thus, Mw would be 1815 for peak 4 in run 2, indicating that polymerization degree is about 6 taking into account that for a poly(isosorbide-co-1,4-BDO carbonate) the molar mass of the repeating unit is 288.
Finally, in order to determine unambiguously that peak 4 corresponds to a poly(isosorbide-co-1,4-BDO carbonate) 20 mL of methyl isobutyl ketone was added to 1.43 g of reaction mixture from run 2 and after stirring for 5 min the mixture was extracted with 3 × 7 mL of water. The organic phase was separated, dried on Na2SO4, filtered, the solvent evaporated at vacuum and the resulting residue dissolved in dried DMF and analyzed by GPC and ATR-FTIR. GPC chromatograms of samples before and after extraction are depicted in Fig. 6. As it can be seen, only the peak 4 of higher molar mass is obtained indicating that the other species has been extracted by water. Its ATR-FTIR compared with that of a polycarbonate oligomer of Mw 409 obtained by reacting dimethyl carbonate and 1,4-BDO according to the procedure previously disclosed in Section 2.3 is depicted in Fig. 7. As it can be observed, both have the peak of a linear carbonate at 1738 cm−1, but there are clear differences in the regions 900–1100 cm−1 and 450–650 cm−1. Poly(1,4-butanediol carbonate) has no peak in the last region unlike spectrum of peak 4. This region (peaks at 461, 531 and 606 cm−1) is especially interesting because it also appears in the spectrum of isosorbide constituting a fingerprint of its presence. In addition, the intensities of the peaks within this region in the spectrum of polymer corresponding to peak 4 are lower than those found in pure isosorbide ATR-FTIR spectrum, but still significant indicating this polymer is rich in isosorbide units, which together with presence of the peak of linear carbonate at 1738 cm−1 allows to conclude that this polymer is a poly(isosorbide-co-1,4-BDO carbonate) showing that IBMC can be used for obtaining polymers such as polycarbonates.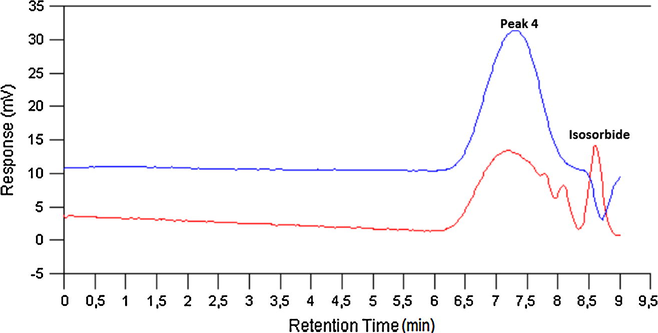
GPC chromatograms of sample from run 2. Red: as obtained after polymerization. Blue: after dissolution in methyl isobutyl ketone and extraction with water.
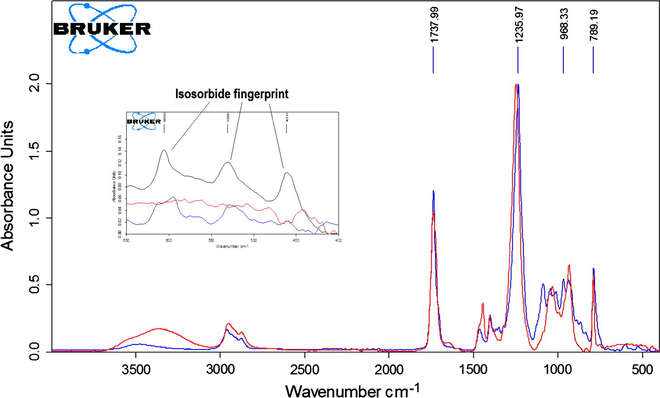
ATR-FTIR spectra of polymers corresponding to peak 4 (blue) and poly(1,4-butanediol carbonate) (red). The spectra within the inserted rectangle show the 450–650 cm−1 region of isosorbide fingerprint, with the black line being pure isosorbide.
4 Conclusions
The synthesis of isosorbide bis(methyl carbonate) (IBMC) by transesterification of isosorbide (IS) with dimethyl carbonate (DMC) has been studied at reflux temperature using cesium and potassium carbonates as catalysts. Continuous removal of methanol for shifting equilibrium to the right and obtaining IBMC in high yields is needed. Reaction rate is higher with cesium carbonate than with potassium carbonate. A competitive reaction is the polymerization of IBMC with the intermediate isosorbide mono(methyl carbonate) and/or isosorbide leading to oligomers formation. Low catalyst concentrations and high IS/DMC molar ratios suppress strongly the synthesis of oligomers so that a 97% IBMC yield is obtained with cesium carbonate as a catalyst at a concentration of 0.2 mol% vs. IS, and a DMC/IS molar ratio of 30. The superior behavior of cesium carbonate is attributed to both its stronger basicity and its partial solubility in the reaction medium unlike potassium carbonate. On one hand, its higher base strength speeds the reaction rate up for IBMC synthesis in a higher extent than that for oligomers formation. On the other hand, its partial solubility justifies a homogeneous catalysis at low catalyst concentration or a mixed homogeneous-heterogeneous catalysis at high catalyst concentration, with the homogenous catalysis speeding further up the reaction rate for IBMC synthesis, thereby contributing to decrease the oligomer contents.
Evidence of the capability of IBMC to be used as a monomer in polymer synthesis has been given by reacting IBMC and 1,4-BDO in bulk at 130–160 °C using cesium carbonate and DBU as catalysts. The number of reactions carried out is not enough for obtaining definitive conclusions about the extension (yield, molar masses and polydispersities) at which the polycondensation can proceed, but it is enough to show that the reaction leads mainly to the formation of a poly(isosorbide-1,4-BDO carbonate) of a weight average molar mass of 1800 g/mol at 130 °C using DBU as a catalyst, although a competing reaction route resulting in isosorbide and other unidentified species coexists. The relative weight of both routes depends on catalyst nature and temperature, opening the way for suppressing the undesired one by a careful optimization study.
A patent claiming the IBMC synthesis has been applied (Ochoa-Gómez et al., 2015).
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Acknowledgments
Thanks are due to the Ministerio de Economía y Competitividad of the Spanish Government for financial support (project CTQ2009-11376 (sub-programme PPQ)). The contribution of Leire Lorenzo-Ibarreta to the experimental work is also gratefully acknowledged.
References
- Abe, T., Saibara, S., Katayama, H., Aoyama, S., Aoi, K., Yokoe, M., Okada, M., 2008. Ion-conductive electrolyte and cell employing the same. US Patent 7,455,935 B2, November 25.
- The neighbouring effect of isosorbide and its epimers in their reactions with dimethyl carbonate. ScienceOpen Res. 2014
- [CrossRef] [Google Scholar]
- Brack, H.-P., Guyader, M., Vermeulen, H., Patrick, D.J., Willemse, M., 2011. Method of making isosorbide polycarbonate. US Patent 7.863,404 B2, January 4.
- Preparation of dialkyl carbonates via the phase-transfer-catalyzed alkylation of alkali metal carbonate and bicarbonate salts. J. Org. Chem.. 1984;49:1122-1125.
- [Google Scholar]
- Cyclic and noncyclic polycarbonates of isosorbide (1,4:3,6-dianhydro-d-glucitol) Macromolecules. 2006;39:9064-9070.
- [Google Scholar]
- Lipase-catalyzed synthesis of isosorbide monoricinoleate: process optimization by response surface methodology. Bioresour. Technol.. 2010;101:8520-8525.
- [Google Scholar]
- European Commission, 2102. Innovating for sustainable growth: a bioeconomy for Europe. COM (2012) 60 final.
- European Commission, 2105. Closing the loop – an EU action plan for the Circular Economy. COM (2015) 614 final.
- Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): a review. Prog. Polym. Sci.. 2010;35:578-622.
- [Google Scholar]
- Fuertes, P., Ibert, M., Josien, E., Tundo, P., Arico, F., 2013. Method for preparing a dialkyl carbonate of dianhydrohexitol. US Patent 8,339,601 B2, March 19.
- “Cesium ion effect” and macrocyclization. A critical review. Org. Prep. Proc. Int.. 1992;24:287-307.
- [Google Scholar]
- Polymers with shape memory effect from renewable resources: crosslinking of polyesters based on isosorbide, itaconic acid and succinic acid. Polym. Int.. 2013;62:709-712.
- [Google Scholar]
- A perspective on emerging polymer technologies for bisphenol-A replacement. Polym. Int.. 2012;61:1485-1491.
- [Google Scholar]
- Ochoa-Gómez, J.R., Gil, S., Maestro, B., Lorenzo, L., Gómez-Jiménez-Aberasturi, O., 2015. Improved method for manufacturing 1,4:3,6-dianhydrohexitol di(alkyl carbonate)s. US Patent Application US-2015-0336978 A1, November 26.
- Isosorbide as a renewable platform chemical for versatile applications – quo vadis? ChemSusChem. 2012;5:167-176.
- [Google Scholar]
- Synthesis and goat pulmonary vasodilatory activity of some novel 1,3,4-oxadiazoles. Arab. J. Chem.. 2011;4:413-418.
- [Google Scholar]
- The Broad Scope of Cesium Salts in Organic Chemistry. Acros Organics. 2011. (accessed 06.06.2016)
- [Google Scholar]
- New developments in dimethyl carbonate chemistry. Pure Appl. Chem.. 2001;73:1117-1124.
- [Google Scholar]
- Resins and additives for powder coatings and alkyd paints, based on renewable resources. J. Coat. Technol. Res.. 2007;4:177-186.
- [Google Scholar]
- <http://www.marketresearchstore.com/report/isosorbide-market-z48041/> (accessed 20.04.2016).
- <http://www.roquette.com/topic_id=11189/article_id=8959/> (accessed 20.04.2016).
Appendix A
Supplementary material
1H NMR spectrum, 1H NMR spectrum magnification between 3.5 and 5.3 ppm showing the values of integrals, assignment of 1H NMR signals, 13C NMR spectrum, homonuclear correlation spectrum COSY and correlation spectrum 1H–13C HMQC for IBMC are depicted in Figs. S1–S6, respectively. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2016.09.017.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







