Translate this page into:
Tailoring crystal size distributions for product performance, compaction of paracetamol
⁎Corresponding author. seyedeh.pishnamazi@ul.ie (Mahboubeh Pishnamazi),
⁎⁎Corresponding author. patrick.frawley@ul.ie (Patrick J. Frawley)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paracetamol crystals often exhibit poor compressibility properties, which results in capping issues. The Particle Size Distribution (PSD) of paracetamol was engineered to improve the compressibility of paracetamol crystals. This was accomplished by growing paracetamol crystals in the presence of additives. The active pharmaceutical ingredient Phenacetin and impurity 4-chloroacetanalide were used to modify the crystal properties of paracetamol. In solution, the phenacetin or 4-chloroacetanalide molecules adsorb onto the paracetamol crystal faces selectively (110 or 011) and inhibit the further growth of the paracetamol crystal and consequently, the paracetamol crystal growth is reduced substantially. For controlling the PSD of crystal to improve the compressibility of paracetamol crystals, a set of cooling crystallization experiments in the presence of additive was designed. According to a statistical experimental design, the cooling rate was the most effective parameter. The PSD was reduced when paracetamol crystallized from the controlled crystallization in the presence of less than 3 mol% of both additives. These smaller particles increased almost four-fold the compressibility of paracetamol in comparison to the commercial material. Moreover, tablets were prepared for each formulation using a direct compaction method. The results illustrated that a higher tablet hardness of paracetamol was achieved by tailoring the paracetamol crystal size distribution. In addition, the tablet disintegration time was higher for the formulation with increased hardness. Overall, this work presents the potential use of structurally similar compounds as additives to alter the mechanical properties of an API.
Keywords
Pharmaceutics
Crystallization
Paracetamol
Particle size distribution
Additives
Compressibility
1 Introduction
Crystallization is the major unit operation in manufacturing solid dosage oral formulations, as the properties of the finished product (e.g. tablets) are highly dependent on the crystal attributes of Active Pharmaceutical Ingredient (API). This processing step selectively allows us to yield a highly pure form of the desired API, while also allowing the tailoring of crystal size and morphology to achieve engineered products. Having control over these aspects becomes important for downstream processes such as isolation, drying, packaging, etc. It is also of great importance to control batch life, stability, tabletting, and ultimately bioavailability/dissolution in the body. Therefore, there is an increased interest in engineering the crystal habit of the pharmaceutical compounds to achieve the desired shape, size and surface area for enhanced bioavailability. Manipulating the API crystal habit has many benefits, such as improving the physicochemical properties of APIs (El-Zhry El-Yafi and El-Zein, 2015). In some cases, impurities are added deliberately to generate desirable crystal shape (York, 1983). It has been proven that additives are prone to alter the mechanical properties of crystals such as flowability and compressibility in pharmaceutical downstream processing (Nokhodchi et al., 2010).
Solvent, temperature and crystallization conditions have impact on formation of a different form of the drug. Particular forms of a drug have different physicochemical properties which have an important influence on how it is processed into a drug product (Chadha et al., 2011). There are three polymorphic forms of paracetamol; the common crystal form (form I, monoclinic) which is used in the pharmaceutical product is thermodynamically stable, whereas the forms II and III are metastable phases, which can undergo solid phase transitions. Form I is prepared in large quantities and described as plate-shape. The tablets produced by form I has high capping tendency because of a stiff construction of the molecules inside the crystal (Krycer et al., 1982; Aguiar et al., 1967; Bauer et al., 2001). However, the form II crystal has a higher water solubility and compressibility advantageous for pharmaceutical applications (Al-Zoubi et al., 2002). Orthorhombic paracetamol (form II) contains slip planes in its crystal structure and, as a result, is able to undergo plastic deformation upon compaction (Joiris et al., 1998). In contrast, the monoclinic lacks slip planes in its crystal structure, which is a requisite for plastic deformation under compaction.
Paracetamol is one of the most common medications worldwide which is a well-known API for poor compressibility. To improve the poor compression behaviour of paracetamol, some researchers alter the form and lattice of crystals, while others focus on changing the shape (habit) of crystals. Owing to the better tabletability of orthorhombic, its bulk crystallization from solution attracts much interest (Šimek et al., 2017). One of the methods that have been reported to bulk production of form II is growing it as a polycrystalline material from the melted form I in a nonoxidizing atmosphere (Di Martino et al., 1996; Di Martino et al., 1997). The scaling-up of this method could be difficult due to the high oxidability of melted paracetamol and the overlap of transition phases of two forms (de Wet et al., 1998). Zoubi et al. to have a better tabletability, studied the crystallization condition to produce the orthorhombic paracetamol form (Al-Zoubi et al., 2002). However, the disadvantage of the orthorhombic form is kinetically stable, so the possible transition to form I (Wang et al., 2011). There are other methods to produce paracetamol with improved compactability properties in laboratory-scale through changing the crystal habit such as modifying the crystallization process, and crystallization in the presence of additives such as polymers (Garekani et al., 2000; Nathan and Scobell, 2012; Shekunov et al., 1997; Had et al., 1996) or by changing the solvent in crystallization method. For example, Fachaux et al. used the desolvation process to produce a crystal structure for Paracetamol similar to the sintered-like crystal structure (Fachaux et al., 1995). Femi-Oyewo studied the effect of additive (agar and gelatin) in the growth medium of paracetamol crystals and showed that the compact hardness and disintegration times of paracetamol was increased in the present of additives (Femi-Oyewo and Spring, 1994). Most recently, Simek et al. employed modified crystallization procedures to obtain plate, irregular and spherical shaped particles from raw paracetamol to prepare directly compressible drug particles. Their results showed a considerable effect of shape alteration and compression force in comparison with the effect of the size modification on tablet compression (Šimek et al., 2017). Current industrial standards rely on adding binders, lubricants and disintegrants such as polyvinylpyrrolidone (PVP), gelatine or starch derivatives to improve the tabletting properties (Di Martino et al., 1996; Di Martino et al., 1997; Fachaux et al., 1995). However, these techniques suffer from common limitations such as lower disintegration time (and thus lower drug release rate) of the solid-dosage forms (Andrews, 2007).
Powder properties are key critical attributes which can influence the pharmaceutical processing method and the final product’s quality (Søgaard et al., n.d.). Crystal size is of great importance in tablet tensile strength and hardness as well (Mohan, 2012). Indeed, increasing the particle size results in a decrease in tensile strength (Rajani et al., n.d.). Various studies have reported the relationship between powder properties such as particle size and compressibility, and tablet properties such as tensile strength (Pharm, 2012; Zuurman et al., 1994; Riepma et al., 1993; Alderborn and Frenning, 2018). In addition, particle size and particle size distribution affect powder compressibility properties, so that smaller particle sizes typically results in higher compressibility of powders and better compaction (Khomane and Bansal, 2013; Iman et al., 2015; Almaya and Aburub, 2008).
In this study, paracetamol crystals were optimized to produce the desired PSD in the presence of additives, which favour tabletting. The focus is to find a suitable additive and best crystallization conditions to alter the PSD of crystals that can produce materials with desirable mechanical properties. Thus, in the current study, the strategy to improve the mechanical properties is modifying the crystallization conditions as well as applying additives to achieve the desired size and shape of crystals. In this study the form of paracetamol crystal (form I) is not changing after introducing the additives, however, the habit of crystals are changing and consequently the better compression behaviour were observed. Also, this allows one to directly compress into (binder-free) high-dosage tablets of a fast-releasing drug. It can reduce or even eliminate the costs involved in powder handling and the costs involved in granulation, milling and drying. Indeed, modification of crystal size distribution of paracetamol (PA) was investigated using phenacetin (Phen) and 4-Chloroacetanilide (CA) (Steendam et al., 2019). In our previous work, the compressibility of PA in presence of CA was studied (Keshavarz et al., 2019). CA is one of the main impurities of PA that is not a hazardous substance and as such can be used as an additive to improve the properties of PA (Almaya and Aburub, 2008). Phenacetin (p-ethoxyacetanilide) and paracetamol (p-hydroxyacetanilide) are more popular analgesic antipyretics. Phenacetin is structurally similar to paracetamol, and is employed as an additive here, due to its availability and low price with no reported polymorphs and it crystallizes as needle-like form (Croker et al., 2015). Therefore, the advantage of this work could be using phenacetine as an additive for improving the crystal habit of paracetamol which has similar medical properties with paracetamol that even can improve medical properties of paracetamol tablet (similar to the combination of paracetamol and ibuprofen) which needs further studies. The chemical structures of the materials used in this work are shown in Fig. 1. Further investigation of tabletting was carried out using direct compaction method to study tablet properties such as hardness and disintegration time.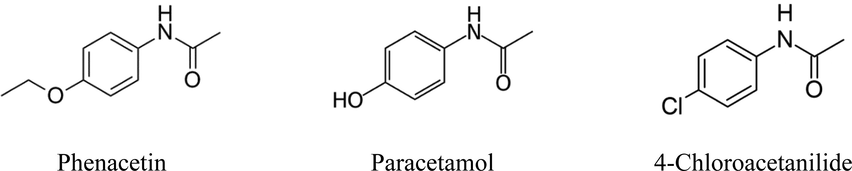
Chemical structures of paracetamol and the two additives phenacetin and 4-chloracetanilide used in this work.
2 Experimental section
2.1 Materials
Paracetamol (98.0–102.0%), phenacetin (99%) and methanol (HPLC grade, 99%) were acquired from Sigma-Aldrich. 4-chloroacetinalide (99%) and 2-propanol (analytical grade, 99.97%) were purchased from Alfa Aesar and Fisher Scientific, respectively. All chemicals purchased were reagent grade (Sigma-Aldrich) and were used without further purification. Milli-Q ultrapure water was used in all the experiments.
2.2 Cooling crystallization
A set of experiments were designed based on fractional factorial method and conducted in a Mettler Toledo Easymax 402 setup. The crystallizer was equipped with a pitched-blade stirrer (ø = 2.5 cm) (Keshavarz et al., 2018; Steendam et al., 2018). Cooling crystallization of paracetamol was performed in the presence and absence of 4-chloroacetinalide and phenacetin as additives. Paracetamol (76 g), 4-chloroacetinalide (2 mol%, 5 mol% and 10 mol%) and phenacetin (2.5 g, 2.8 mol%) were mixed (400 rpm) with 2-propanol (240 g) as solvent and heated to 70 °C and hold for 1 h for full dissolution. After dissolving, the solution cooled down to 15 °C with a cooling rate of 0.9 K/min to reach a supersaturation ratio S = 2.8 (The solubility measurements for the additives have been reported in the Supporting File, SI) (Keshavarz, 2019).
2.3 Compressibility study
FT4 powder flow rheometer (Freeman Technologies, UK) was used to measure the compressibility of powders (Pishnamazi et al., 2019). A vented piston was used as a standard measurement method to compact the powder by applying a normal stress. During the test, the range of normal stress was varied between 1 and 2–4–6–8–10–12–15 kPa (Keshavarz, 2019).
2.4 Tablets preparation
The tablets for each formulation were prepared via direct compaction method. A single-punch tablet press apparatus (Gamlen Tableting GTD-1 D series, UK) was employed. For the preparation of the tablets, 100 mg of each blend was considered to compact in a 6 mm die. The tablet press was set at a fixed load mode with the load of 400 kg and the compaction rate was fixed at 180 mm/min (Keshavarz, 2019).
2.5 Tablet tensile strength
The influence of compressibility on tablet properties was evaluated by the tablet tensile strength measurements using Pharma Test, PTB 311E, Hainburg, Germany. This test determines the mechanical strength of the tablets and influence on tablet disintegration time and drug release rate (Keshavarz, 2019; Pishnamazi et al., 2019; Pishnamazi et al., 2019).
2.6 Tablet disintegration time
Pharma Test PTZ-DIST- Disintegration Test Instrument (Hainburg, Germany) was used to study tablet disintegration times. 900 mL of deionized water was used to fill the apparatus chamber. The apparatus was adjusted to 100 rpm and the water temperature was kept constant at 37 °C. For each formulation, three samples were tested. The disintegration test was conducted until the tablet was completely disintegrated in water (Keshavarz, 2019; Pishnamazi et al., 2019; Pishnamazi et al., 2019).
2.7 Crystal size and shape analysis
The particle size distribution analysis was carried on Malvern Mastersizer 3000 along with a wet dispersion unit. An absorption index of 0.1 for paracetamol was used, as per the CAS datasheet. Cyclohexane was used as non-solvent and a stirring speed of 2500 rpm was applied to ensure a sufficiently mixed suspension (Steendam et al., 2018). Refractive indices of 1.619 and 1.426 were set for paracetamol and cyclohexane, respectively. 1 g sample of the dried crystalline product was dispersed in cyclohexane and enter the flow cell of the Mastersizer unit. First, the laser alignment was adjusted and when a stable background signal was recorded then the sample added. The results reported by Mastersizer in this work are the average value of three performed measurements.
Scanning electron microscopy (SEM) images were taken utilizing a JEOL Carryscope. Before analysing, the crystalline samples were coated with a thin layer of gold.
3 Results and discussion
3.1 Influence of CA as additive on paracetamol crystal
An aspect ratio (or elongation factor) is a method to describe the crystal habit (Borsos and Lakatos, 2014). The aspect ratio is defined as the ratio of the width to the length of the particle as:
The aspect ratio values are within 0–1. It has already been shown that the crystal habit of paracetamol alters in the presence of CA (Keshavarz et al., 2019).
Cooling crystallization experiments were performed in the presence of different amounts of CA (10 mol%, 5 mol%, 2 mol% and without impurity) between 60 °C and 15 °C with the cooling rate of 0.9 K/min and stirring rate of 400 rpm. Fig. 2 provides the images of PA crystallized in presence of different amounts of CA. The aspect ratio of recrystallized samples in the presence of CA increases with the amount of CA in the solution (shown in Fig. 2) (Keshavarz et al., 2019). The PA crystallized in the presence of 2 mol% (1.7 g) of CA showed the smaller size of crystals, but more than this amount led to the needle shape crystals. The needle shape crystals are troublesome in the subsequent processing steps required in the drug production. Therefore, the experimental design approach was adopted to analyse the effect of crystallization conditions on the PSD of PA crystals in the presence of 2 mol% (1.7g) of CA.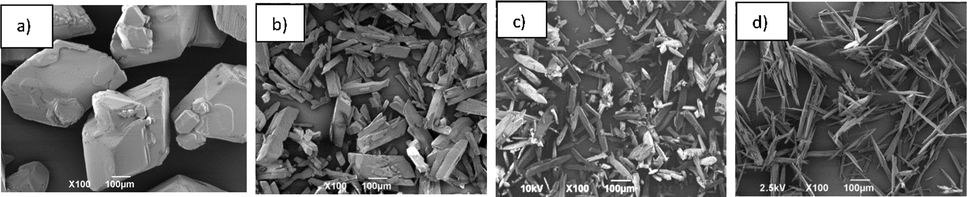
SEM images of product crystals obtained from cooling crystallization experiments involving PA: a) no additive, b) 1.7 g of CA additive, c) 4.25 g of CA additive and d) 8.5 g of CA additive. A cooling rate of 0.9 K/min and the stirring rate of 400 rpm were applied for the experiments. The scale bar represents 100 μm.
To analyse the results of experiments in this work, D50 is also considered as output which is one of the most meaningful terms for particle size distributions. Median value (D50) is defined as the size that splits the distribution with half above this diameter and half below. Particle sizes have a significant effect on tabletting and granulation processes. On the one hand, small particles help dissolution especially for a drug with poor water solubility (like paracetamol), but too small particles are more sensitive to over-compression as it can lead to hard tablets which hardly disintegrate. Large particles, on the other hand, lead to better flowability. However, for the tabletting process with large particles, filling the interstitial spaces between the larger particles is challenging. Generally, homogeneous distribution and narrow particle size distribution is preferable.
The design of experiment (DoE) technique was employed to investigate the effect of the crystallization process conditions on the size and shape of paracetamol crystals. Particle shape is quantified using the aspect ratio and particle size is represented by D50. The influence of four factors (cooling method, cooling rate, seeding and stirring rate) on the crystal habit quantified by performing experiments are presented in Table 1. PA percentage in the PA crystallized for the experiments listed in Table 1 reported in Table S3 (SI).
No.
Cooling method
Cooling rate [K/min]
Stirring rate [rpm]
Seeding
Mean AR
D50
1
Linear
0.9
300
No
0.31
363.6
2
T-cycle
0.9
300
No
0.35
355.7
3
Linear
0.1
300
No
0.14
692.1
4
Linear
0.9
400
No
0.35
261.5
5
T-cycle
0.9
400
No
0.45
436
6
Linear
0.1
400
No
0.15
585
7
T-cycle
0.1
400
No
0.15
875
8
Linear
0.9
300
Yes
0.4
470
9
T-cycle
0.1
300
Yes
0.07
649
10
Linear
0.9
400
Yes
0.31
274.4
11
T-cycle
0.9
400
Yes
0.32
405.6
12
Linear
0.1
400
Yes
0.16
840
13
T-cycle
0.1
400
Yes
0.12
796.3
To have a better understanding of the results of experiments performed in this work, Fig. 3 illustrates the data listed in Table 1. Fig. 3. Shows that the experiments number 1, 2, 4, 5, 8, 10 and 11 which performed in the fast cooling rate (in the right side of the plot with circle symbols) have a smaller size and lower mean AR in comparison with the slow cooling rate which applied on the experiment number 3, 6, 7, 9, 12 and 13 (in the right side of the plot with rectangular symbols).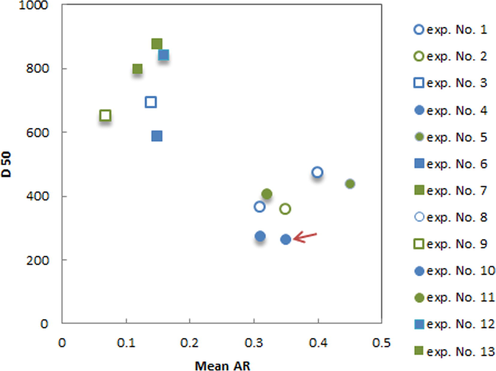
Effect of experimental condition on D50 versus mean Aspect ratio for the experiments listed in Table 1, slow cooling rate (□), fast cooling rate (○), fast cooling rate (filled symbols), slow cooling rate (empty symbols), seeded (symbols with shadow) and without seeding (symbols without shadow). The red arrow shows the preferred size.
Fig. 4 indicates SEM images of recrystallized PA crystals in the presence of 2 mol% CA from experiments conducted at two different cooling rates (0.1 and 0.9 K/min). Table 1, Fig. 3. and Fig. 4 indicate PA crystallized with the slow cooling rates resulted in larger crystals (D50: 585–875 µm) with rod-like/needle-like habits PA crystals (AR: 0.07–0.16). Furthermore, the fast cooling rates cause a lower D50 value (261.5–470 µm) and a larger aspect ratio (0.31–0.45). Crystals of PA with large needle shapes appeared in slow cooling rate and this is expected as it is thermodynamically favourable to grow larger crystals.
SEM images of PA samples isolated from experiments at different crystallization conditions. Scale bars represent 500 μm. Numbers denote experiments from Table 1 (first row, from left to right: experiment no. 4, no. 5 and no. 2.; second row from left to right: experiment no. 6, no. 3 and no. 7).
To analyse the effect of each factor on the AR and D50, Pareto graph (De Souza et al., 2016) using Minitab 18 was constructed (Fig. 5). The factor that exceeds the vertical line corresponds to an effect which is statistically significant at 95% confidence level in terms of the change of the analysed response. Based on the Pareto chart, the crystal shape and size of PA crystals is strongly affected by the cooling rate as the most significant factor. As mentioned earlier, large size and needle shape crystals are not preferable sizes for paracetamol crystal to avoid difficulty in the tabletting process. Although the paracetamol crystals from fast cooling rate form I (see SI), they have a similar shape as form II which is preferable for the tabletting process. Therefore, the linear fast cooling rate without seeding (experiment no. 4 which indicates with an arrow in Fig. 3) was selected as the optimum condition to make the smaller particle size of modified PA crystals. Similar experimental conditions (No seeding, 400 rpm and 0.9 K/min) were applied for the crystallization of PA in the presence of Phenacetin.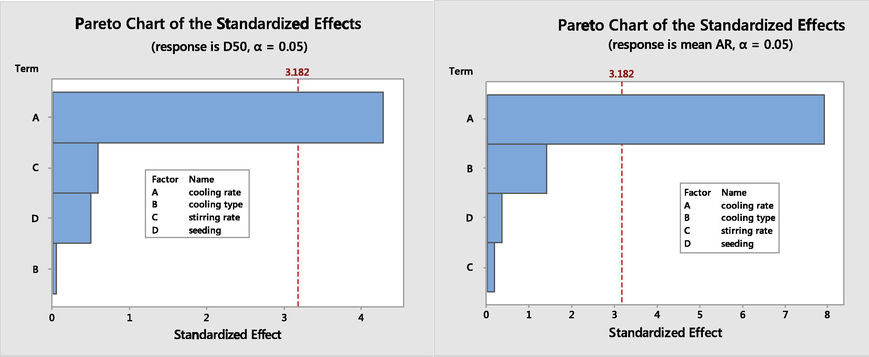
Pareto chart of the effects from the tested factors on the PA crystals in the presence of 1.7 g CA; left: where the response is the D50, right: where the response is the AR. Effects that pass the vertical dashed line at 3.18 are statistically significant.
3.2 Influence of phenacetin as additive on paracetamol crystal
SEM images of recrystallized paracetamol samples in presence of and without phenacetin are shown in Fig. 6. It is illustrated that the samples recrystallized in the presence of 2.5 g phenacetin possess smaller crystal size. Less than 2.5 g did not influence the size and compressibility of PA crystals. Crystallite packing can change crystal size and therefore determine mechanical behaviour.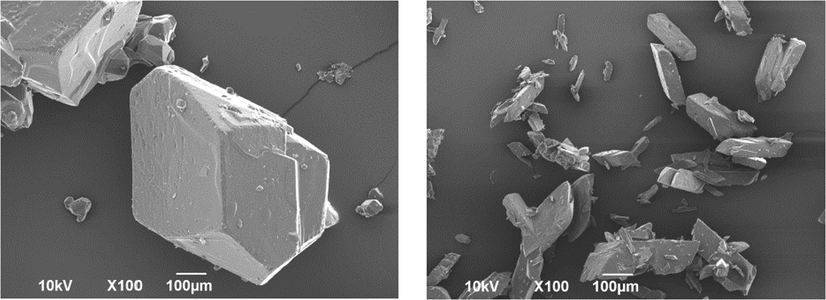
Scanning electron micrographs of untreated (left), and treated PA powders in presence of 2.5 g Phen (right). Cooling crystallization conditions: No seeding, 400 rpm and 0.9 K/min.
3.3 Particle size distributions
PSD of particles produced in manufacturing processes is very important for process and product development because it influences the design of downstream processes and physicochemical properties like compressibility and tabletability (Stone et al., 2009). For poorly water-soluble APIs, small average particle size with a narrow particle size distribution is preferred. Fig. 7 shows recrystallized paracetamol particles in the presence of both additives (CA or phenacetine) lead to narrower PSD and smaller crystals, which favour better content uniformity. Less than 3 mol% of both additives change the average crystal size approximately from 600 µm to 200 µm.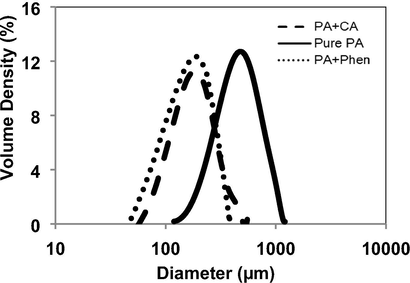
Particle size distribution of PA samples isolated from untreated (solid line) and treated PA powders in the presence of 1.7 g CA (dashed line) and in the presence of 2.5 g Phen (dotted line). Cooling crystallization conditions: No seeding, 400 rpm and 0.9 K/min.
In general, the structurally similar additives or impurities adsorb on the surface of the crystals [44]. In the present case, Phenacetin and 4-chloroacetanalide may have higher binding energy/affinity towards the paracetamol (due to the structural similarity) which can affect the crystal habit remarkably. In solution, the phenacetin or 4-chloroacetanalide molecules adsorb onto the paracetamol crystal faces selectively (110 or 001) and obstructs the further growth of the paracetamol crystal and consequently, the paracetamol crystal growth reduces substantially. Fig. 8 shows the growth of paracetamol crystal (form I) along a and c directions and formed slip planes (see Fig. 9). Crystallization of paracetamol in the presence of additive changed the morphology of paracetamol which was perceived to be a reason for the improved compressibility in the present case (Keshavarz et al., 2019). This may be the reason for getting smaller particle size crystals in the presence of these impurities or additives.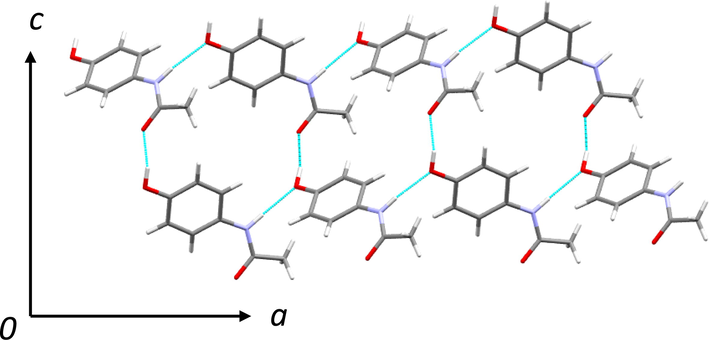
Crystal structure of paracetamol form I, growing along a and c directions.
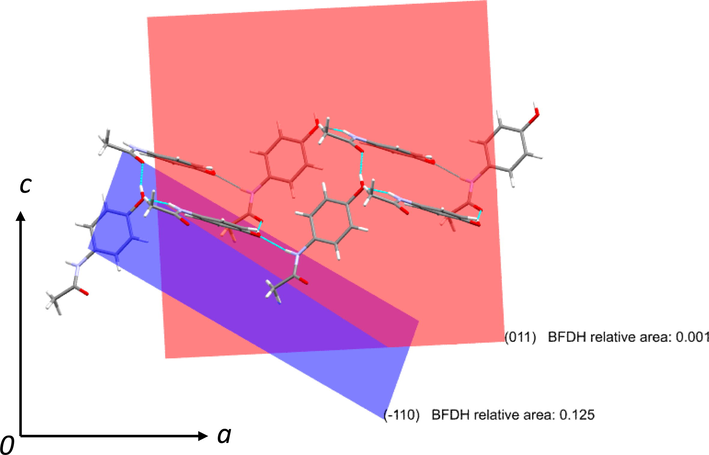
Crystal growth parallel to a c plane and expected growth along (−1 1 0) and (0 1 1).
3.4 Effect of additives on the compressibility of crystallized paracetamol
The compressibility of different paracetamol formulations was analysed using FT4 powder rheometer. The results show that the compressibility of pure PA was 7.26%, while it was increased to 27.22% by adding 2.5 g Phen to the initial crystallization solution and to 28.2% by adding 1.7 g CA to the initial crystallization solution (see Fig. 10).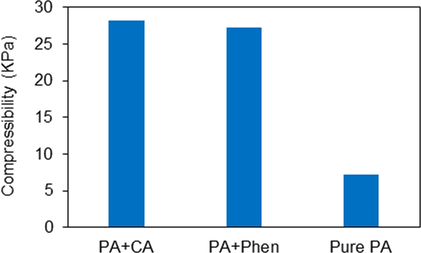
Compressibility of different formulations of paracetamol, PA crystallized in presence of 1.7 g CA, PA crystallized in the presence of 2.5 g Phen and PA crystallized without additive. Cooling crystallization conditions: No seeding, 400 rpm and 0.9 K/min.
3.5 Effect of additives on tablet tensile strength and disintegration time
The tensile strength of the prepared tablets was measured for different formulations of paracetamol and the results are presented in Fig. 11 (a). All tablets are in the same size and the tablet tensile strength is equal to tablet hardness in this study. It can be seen that higher tensile strength levels have been obtained for formulations containing paracetamol with CA as an additive, which is due to its higher compressibility and smaller particle size distribution. Paracetamol tablet tensile strength was increased 7% by adding 1.7 g CA to the initial crystallization solution and increased by 9% with the addition of 2.5 g Phen to the initial paracetamol solution. In addition, higher tensile strength or hardness results in longer disintegration times as shown in Fig. 11 (b) and this can be attributed to better compaction properties of these formulations.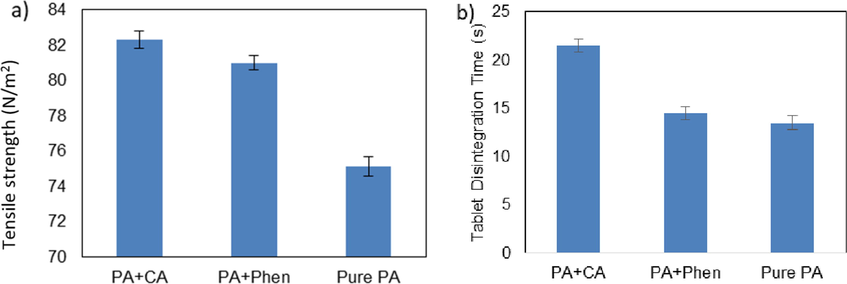
Tablet hardness (a) and disintegration time (b) for different formulations of PA; PA crystallized in presence of 1.7 g CA, PA crystallized in the presence of 2.5 g Phen and PA crystallized without additive. Cooling crystallization conditions: No seeding, 400 rpm and 0.9 K/min.
4 Conclusions
Having control of the PSD allows manufacturers to avoid an additional unit process in the form of milling, which is a mechanical method of producing the desired crystal size. Also, a lack of uniformity in PSD can have consequences on downstream processes such as filtering and drying. It also impacts the efficacy of the API. In this work, paracetamol crystal sizes were influenced and successfully modified by additives 4-Chloroacetanilide and phenacetin. The structurally similar additives were chosen because they can adsorb on the surface of paracetamol crystals to stop the growth and consequently lead to the smaller paracetamol crystals. Phenacetin or 4-chloroacetanalide molecules adsorb onto the paracetamol crystal faces selectively (110 or 001) and stops the further growth of the paracetamol crystal and consequently, the paracetamol crystal growth is reduced. Growth of crystals depends on the crystallization conditions. PSD was found smaller and narrower when paracetamol crystallized from the controlled crystallization in the presence of both additives. Compressibility tests showed that the presence of additives significantly improves the compressibility behaviour of paracetamol powder. This led to better compaction during the tabletting process, and tablet properties were affected by smaller particle size distribution. Lastly, owing to the similar medical properties of phenacetine to the paracetamol, the addition of phenacetin not only improves the tabletting properties of paracetamol, but also may improve the medical properties of paracetamol.
Acknowledgements
The authors are grateful to Synthesis and Solid-State Pharmaceutical Centre (SSPC) for the financial support of this project. S. Shirazian acknowledges the supports by the Government of the Russian Federation (Act 211, contract 02. A03.21.0011) and the Ministry of Science and Higher Education of the Russian Federation (grant FENU-2020-0019).
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J. Pharm. Sci.. 1967;56(7):847-853.
- [CrossRef] [Google Scholar]
- Alderborn, G., Frenning, G., 2018. Compressibility and tablet forming ability of bimodal granule mixtures: Experiments and DEM simulations 540, 120–131. https://doi.org/10.1016/j.ijpharm.2018.02.006.
- Effect of Particle Size on Compaction of Materials with Different Deformation Mechanisms with and without Lubricants. AAPS PharmSciTech.. 2008;9(2):414-418.
- [CrossRef] [Google Scholar]
- Crystallization conditions and formation of orthorhombic paracetamol from ethanolic solution. J. Pharm. Pharmacol.. 2002;54:325-333.
- [CrossRef] [Google Scholar]
- Advances in solid dosage form manufacturing technology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci.. 2007;365(1861):2935-2949.
- [CrossRef] [Google Scholar]
- Ritonavir: An extraordinary example of conformational polymorphism. Pharm. Res.. 2001;18:859-866.
- [CrossRef] [Google Scholar]
- Investigation and simulation of crystallization of high aspect ratio crystals with fragmentation. Chem. Eng. Res. Des.. 2014;92(6):1133-1141.
- [CrossRef] [Google Scholar]
- Crystal habit, characterization and pharmacological activity of various crystal forms of arteether. Acta Pharm. Sin. B.. 2011;1(2):129-135.
- [CrossRef] [Google Scholar]
- Demonstrating the Influence of Solvent Choice and Crystallization Conditions on Phenacetin Crystal Habit and Particle Size Distribution. Org. Process Res. Dev.. 2015;19(12):1826-1836.
- [CrossRef] [Google Scholar]
- Evidence of Crystal Nuclei Breeding in Laboratory Scale Seeded Batch Isothermal Crystallisation Experiments. Cryst. Growth Des. Evid.. 2016;16:3443-3453.
- [CrossRef] [Google Scholar]
- A study of the changes during heating of paracetamol. Drug Dev. Ind. Pharm.. 1998;24(5):447-453.
- [CrossRef] [Google Scholar]
- A new pure paracetamol for direct compression: The orthorhombic form. Int. J. Pharm.. 1996;128(1-2):1-8.
- [CrossRef] [Google Scholar]
- Preparation and physical characterization of forms II and III of paracetamol. J. Therm. Anal.. 1997;48(3):447-458.
- [CrossRef] [Google Scholar]
- Technical crystallization for application in pharmaceutical material engineering: Review article. Asian J. Pharm. Sci.. 2015;10(4):283-291.
- [CrossRef] [Google Scholar]
- Pure Paracetamol for direct compression Part I. Development of sintered-like crystals of Paracetamol. Powder Technol.. 1995;82(2):123-128.
- [CrossRef] [Google Scholar]
- Studies on paracetamol crystals produced by growth in aqueous solutions. Int. J. Pharm.. 1994;112(1):17-28.
- [CrossRef] [Google Scholar]
- Highly compressible paracetamol - II. Compression properties. Int. J. Pharm.. 2000;208(1-2):101-110.
- [CrossRef] [Google Scholar]
- Had, B.Y., Garekani, A., October, P., 1996. The Characterization and Compaction Properties of Manipulated Paracetamol Crystals By.
- The Effect of Particle Size on Compressibility of Cinnamon Granules. J. Appl. Ind. Sci.. 2015;3:150-153.
- [Google Scholar]
- Compression behavior of orthorhombic paracetamol. Pharm. Res.. 1998;15:1122-1130.
- [CrossRef] [Google Scholar]
- The Importance of Impurity on Pharmaceutical Processes. University of Limerick; 2019.
- Impact of Mother Liquor Recycle on the Impurity Buildup in Crystallization Processes. Org. Process Res. Dev.. 2018;22(11):1541-1547.
- [CrossRef] [Google Scholar]
- Influence of Impurities on the Solubility, Nucleation, Crystallization, and Compressibility of Paracetamol. Cryst. Growth Des.. 2019;19(7):4193-4201.
- [CrossRef] [Google Scholar]
- Effect of Particle Size on In-die and Out-of-die Compaction Behavior of Ranitidine Hydrochloride Polymorphs. AAPS PharmSciTech.. 2013;14(3):1169-1177.
- [CrossRef] [Google Scholar]
- The prediction of paracetamol capping tendencies. J. Pharm. Pharmacol.. 1982;34(12):802-804.
- [Google Scholar]
- Compression Physics of Pharmaceutical Powders: A Review. Int. J. Pharm. Sci. Res.. 2012;3:1580-1592.
- [Google Scholar]
- Physico-mechanical and dissolution behaviours of ibuprofen crystals crystallized in the presence of various additives. Daru.. 2010;18:74-83.
- [Google Scholar]
- Pharm, A., 2012. A compressibility and compactibility study of real tableting mixtures: The effect of granule particle size 62, 325–340. https://doi.org/10.2478/v10007-012-0028-8.
- Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol.. 2019;341:38-50.
- [CrossRef] [Google Scholar]
- Design of controlled release system for paracetamol based on modified lignin. Polymers (Basel).. 2019;11:1-10.
- [CrossRef] [Google Scholar]
- Effect of lignin on the release rate of acetylsalicylic acid tablets. Int. J. Biol. Macromol.. 2019;124:354-359.
- [CrossRef] [Google Scholar]
- Rajani, C., Kumar, D.D., Jaya, D., Kumar, J.A., n.d. Effects of granule particle size and lubricant concentration on tablet hardness containing large concentration of polymers.
- Riepma, K.A., Vromans, H., Zuurman, K., Lerk, C.F., 1993. The effect of dry granulation on the consolidation and compaction of crystalline lactose 97, 29–38.
- In situ optical interferometric studies of the growth and dissolution behavior of paracetamol (Acetaminophen) crystals. 3. Influence of growth in the presence of p-acetoxyacetanilide. J. Phys. Chem. B.. 1997;101(44):9107-9112.
- [CrossRef] [Google Scholar]
- Comparison of compression and material properties of differently shaped and sized paracetamols. KONA Powder Part. J.. 2017;2017:197-206.
- [Google Scholar]
- Søgaard, S.V., Bryder, M., Allesø, M., Rantanen, J., n.d. Characterization of powder properties using a powder rheometer, 1–8.
- Effects of Scale-Up on the Mechanism and Kinetics of Crystal Nucleation. Cryst. Growth Des. Eff.. 2018;18(9):5547-5555.
- [CrossRef] [Google Scholar]
- Thermodynamic properties of paracetamol impurities 4-nitrophenol and 4″-chloroacetanilide and the impact of such impurities on the crystallisation of paracetamol from solution. J. Chem. Thermodyn.. 2019;133:85-92.
- [CrossRef] [Google Scholar]
- Implementation and use of robust refinement in powder diffraction in the presence of impurities. J. Appl. Crystallogr.. 2009;42(3):385-391.
- [CrossRef] [Google Scholar]
- Polymorph transformation in paracetamol monitored by in-line NIR spectroscopy during a cooling crystallization process. AAPS PharmSciTech.. 2011;12(2):764-770.
- [CrossRef] [Google Scholar]
- Solid-state properties of powders in the formulation and processing of solid dosage forms. Int. J. Pharm.. 1983;14(1):1-28.
- [CrossRef] [Google Scholar]
- The relationship between bulk density and compactibility of lactose granulations. Int. J. Pharm.. 1994;102(1-3):1-9.
- [Google Scholar]
Appendix A
Supplementary material
The measured and calculated solubility of all components along with SEM images of recrystallized phenacetin, and XRD diffraction patterns are reported in the supporting file. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103089.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







