Translate this page into:
Tailoring of novel biologically active molecules based on N4-substituted sulfonamides bearing thiazole moiety exhibiting unique multi-addressable biological potentials
⁎Corresponding authors at: Department of Chemistry, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. amhfarghaly@uqu.edu.sa (Essam M. Hussein), saahmed@uqu.edu.sa (Saleh A. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nowadays, the growth of drug-resistant microbial strains (MDRs) is a serious public health threat worldwide. Moreover, tens of millions of people are annually diagnosed with cancer worldwide, and more than half of patients ultimately die. In the present study, a new series of 2-(4-substituted-thiazol-2-ylamino)acetamides and N-(4-substituted-thiazol-2-yl)acetamides incorporating sulfonamide moieties were designed, synthesized, well-characterized and successfully evaluated for their antimicrobial activity against multidrug resistant strains and screened for cytotoxic activity against normal lung fibroblast (WI-38), human lung carcinoma (A549), and human breast carcinoma (MDA-MB-231) cell lines. Fluorescence-activated cell sorting (FACS) analysis and molecular modeling study were performed to identify the mode of action of the novel synthesized compounds and their binding interactions with the active sites of dihydrofolate reductase enzyme (DHFR).
Keywords
Sulfonamide
Anticancer
Antimicrobial
Fluorescence-activated cell sorting (FACS)
Thiazole
Multidrug resistant strains
Molecular docking
DHFR inhibitors
1 Introduction
Over past decades, sulfonamides have been found to be associated with comprehensive and broad spectrum of biological activities embracing antibacterial (Drews, 2000), antifungal (Krátký et al., 2012), anti-carbonic anhydrase (Supuran and Scozzafava, 2001), hypoglycemic (Anjaneyulu et al., 1980), diuretic (Supuran and Scozzafava, 2000; Jaiswal et al., 2004), anti-human immunodeficiency virus (HIV) (Ogden and Flexner, 2001) and anti-thyroid (Thornber, 1979). Additionally, a large number of structurally inventive sulfonamides have recently been reported to display substantial in-vitro and in-vivo antitumor activity (Al-Said et al. 2010; Alqasoumi et al., 2010a; Alqasoumi et al., 2010b; Ghorab et al., 2011; Ghorab et al., 2012). The antitumor activity is accomplished by the sulfonamides through dissimilar of mechanisms, such as histone deacetylases (HDACs) inhibition (Payne et al., 2008), cell cycle arrest in the G1 phase (Fukuoka et al., 2001), NADH oxidase inhibition (Villar et al., 2004), carbonic anhydrase (CA) inhibition (Supuran et al., 2001), matrix metalloproteinase (MMPs) inhibition (Casini et al., 2002), cyclin-dependent kinase (CDK) inhibition (Huang et al., 2006), methionine aminopeptidases (MetAPs) inhibition (Kawai et al., 2006), binding to β-Tubulin, and disruption of microtubule assembly (Kenneth et al., 2006).
Furthermore, thiazole ring as a core structural pattern found in a variety of biologically and pharmacologically active molecules, also, it is a structural constituent of natural products such as thiamine (vitamin B1) and penicillin. In addition, thiazole derivatives demonstrated a broad spectrum of medicinal and biological activities, including antiviral (Spector et al., 1998), antimicrobial (Bharti et al., 2010) anti-inflammatory (Yang et al., 2010), antimalarial (Diego et al., 2011), anti-HIV (Bell et al., 1995) and anticancer (Dos Santos et al., 2016; Silva et al., 2017; de Santana et al., 2018) activities. As an epitome heterocyclic amines, 2-aminothiazoles and their derivatives are used as key intermediates for the synthesis of plentiful biologically active compounds, such as biocides, fungicides, sulfur drugs, and as intermediates in the synthesis of numerous antibiotics, where a huge number of 2-aminothiazoles have been substituted with different groups for pharmaceutical applications (Papadopoulou et al., 2005; Geronikaki et al., 2009).
On the other hand, organic molecules with acetamide linkage exhibited miscellany of applications, which are well described. Also, the acetamides and their analogs are well scrutinized as chemotherapeutic agents (Mccarthy et al., 2009; Liu et al., 2012). The acetamide functional group is responsible for urease inhibitory activities (Gull et al., 2016), antimicrobial (Berest et al., 2011), antioxidant (Autore et al., 2010; Ley and Bertram, 2001), anti-inflammatory (Raghavendra et al., 2012) and platelet aggregation inhibitory (Xiang et al., 2018).
The sulfonamide group linked with acetamide moiety bearing different aryl, heteroaryl substituents exhibits enormous pharmacological potency, particularly sulfonamide derivatives encompassing short amine fragments reveal promising anticancer activity (Alsaid et al., 2011; Hussein et al., 2019). Moreover, its honesty to mention that, the pronounced significance of sulfonamide derivatives bearing thiazole moiety in medicinal chemistry cannot be disregarded and the compounds are well-known as pharmaceutical agents and have acquired great interest due to their miscellaneous biological activities. The sulfonamide-thiazole derivatives reveled a huge number of biological activities, such as antimicrobial (Argyropoulou et al., 2009), anticancer (Naaz et al., 2018) and carbonic anhydrase (CA) inhibitors (Kılıcaslan et al., 2016).
Dihydrofolate reductase enzyme (DHFR) is a key enzyme in the process of nucleic acid synthesis in both human and bacteria. This enzyme is accountable for catalysis of the reduction of folate or dihydrofolate to tetrahydrofolate using NADPH. This function made of the DHFR is considered as an important target for different antibacterial and cancer agents (Blakley, 1984; Brown and Kraut, 1992; Hawser et al., 2006).
The interesting and important of the above mentioned findings, motivated and encouraged us to explore design and synthesis of new series of sulfonamide-N4-acetamides bearing thiazole moiety to evaluate their antimicrobial activities against multidrug resistant strains (MDRs) and anticancer activity against A549 lung cancer cells and MDA-MB-231 breast cancer cells as continuation of our research interest which deals with the synthesis of new bioactive nitrogen-containing heterocycles (Hussein et al., 2019; Hussein and Abdel-Monem, 2011; Abdel-Mohsen and Hussein, 2014; Hussein, 2015; Hussein et al., 2015a; Hussein et al., 2015b; Al-Shareef et al., 2016).
2 Experimental
2.1 Chemistry
2.1.1 General methods
Melting points were determined on a Stuart SMP3 melting point apparatus and are uncorrected. FT-IR spectra were recorded on a Shimadzu IR-3600 FT-IR spectrometer in KBr pellets. NMR spectra were acquired on a Bruker Avance 400 instrument (400 MHz for 1H, 100 MHz for 13C) in CDCl3 and DMSO‑d6 solutions, using residual solvent signals as internal standards. All solvents used purchased from Sigma-Aldrich are spectroscopic grade and used without further purifications. 2-Aminothiazole derivatives, namely; 2-aminothiazole (3a), 2-amino-4-(4-bromo-phenyl)thiazole (3b), 2-amino-4-(2-fluorenyl)thiazole (3c), 2-amino-4-(2,7-dichloro-4-fluorenyl)thiazole (3d), 2-amino-4-(1-pyrenyl)-thiazole (3e) and 2-amino-4-phenylthiazole (3f) were prepared accordingly as the reported method (Hantzsch and Weber, 1887). 4-(Piperidin-1-ylsulfonyl)aniline (5a), 4-(morpholin-4-ylsulfonyl)aniline (5b), 2-chloro-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (6a) and 2-chloro-N-(4-(morpholinosulfonyl)phenyl)-acetamide (6b) were prepared according to our previously reported procedure (Hussein, 2015; Hussein et al., 2019).
2.1.2 General procedure for synthesis of 2-chloro-N-(4-substituted-thiazol-2-yl)acetamides 4a-f
A mixture of different 2-amino thiazole derivatives 3a-f, namely; 2-aminothiazole, 2-amino-4-(4-bromo-phenyl)thiazole, 2-amino-4-(2-fluorenyl)thiazole, 2-amino-4-(2,7-dichloro-4-fluorenyl)thiazole, 2-amino-4-(1-pyrenyl)thiazole and 2-amino-4-phenylthiazole (0.1 mol) and chloroacetyl chloride (8.0 mL, 0.1 mol) in dimethylformamide (20 mL) was stirred at room temperature for 2–4 h. The reaction mixture was poured onto ice-water, the obtained solid was filtered off and crystallization from ethanol to give 4a-f.
2.1.3 General procedure for synthesis of 2-(4-substituted-thiazol-2-ylamino)-N-(4-(piperidin-1-ylsulfonyl)-phenyl)acetamides 7a-f and N-(4-(morpholinosulfonyl)phenyl)-2-(4-substituted-thiazol-2-ylamino)acetamides 7g-l
A mixture of chloro compound 6a or 6b (0.001 mol) and different 2-aminothiazole derivatives 3a-f namely; 2-aminothiazole, 2-amino-4-(4-bromophenyl)thiazole, 2-amino-4(2-fluorenyl)-thiazole, 2-amino-4-(2,7-dichloro-4-fluorenyl)thiazole, 2-amino-4-(1-pyrenyl)thiazole and 2-amino-4-phenylthiazole (0.001 mol) in absolute ethanol (10 mL) was refluxed for 5–8 h. The reaction mixtures were concentrated under reduced pressure, the obtained solid was filtered, washed with n-hexane, dried and recrystallized from ethanol to give the titled products 7a-f and 7g-l.
2.1.3.1 N-[4-(piperidine-1-sulfonyl)phenyl]-2-(thiazol-2-ylamino)acetamide (7a)
Pale yellow crystals; yield 53%; mp 95–98 °C; FT-IR = 3328 (NH), 3205 (NH), 3041 (CH arom.), 2940 (CH aliph.), 1699 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.92 (s, 1H, NH), 9.78 (s, 1H, NH), 7.86 (d, J = 8.0 Hz, 2H, Ph-H), 7.70 (d, J = 8.0 Hz, 2H, Ph-H), 7.44 (d, J = 8.0 Hz, 1H, thiazole-H), 7.03 (d, J = 8.0 HZ, 1H, thiazole-H), 4.34 (s, 2H, CH2), 2.85–2.83 (m, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.35–1.32 (m, H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 169.6 (C⚌O), 165.8 (C⚌N), 143.0 (C), 131.4 (CH), 130.1 (C), 129.2 (CH), 119.6 (CH), 107.3 (CH), 47.1 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2.1.3.2 2-(4-Phenylthiazol-2-ylamino)-N-[4-(piperidine-1-sulfonyl)phenyl]acetamide (7b)
Pale yellow crystals; yield 78%; mp 100–102 °C; FT-IR = 3339 (NH), 3110 (NH), 3046 (CH arom.), 2850 (CH aliph.), 1695 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.82 (s, 1H, NH), 7.85 (d, J = 8.0 Hz, 2H, Ph-H), 7.79 (d, J = 4.8 Hz, 2H, Ph-H), 7.71 (d, J = 8.0 Hz, 2H, Ph-H), 7.38–7.35 (m, 2H, Ph-H), 7.11–7.09 (m, 1H, Ph-H), 7.00 (s, 1H, thiazole-H), 4.33 (s, 2H, CH2), 2.86–2.84 (m, 4H, 2CH2), 1.54–1.51 (m, 4H, 2CH2), 1.35–1.31 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.7 (C⚌O), 165.8 (C⚌N), 150.1 (C), 142.9 (C), 135.2 (C), 130.3 (C), 129.2 (CH), 128.9 (CH), 127.6 (CH), 126.0 (CH), 119.6 (CH), 101.9 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2.1.3.3 2-[4-(4-Bromophenyl)-thiazol-2-ylamino]-N-[4-(piperidine-1-sulfonyl)phenyl]acetamide (7c)
Pale yellow powder; yield 76%; mp 144–145 °C; FT-IR = 3284 (NH), 3110 (NH), 3025 (CH arom.), 2940 (CH aliph.), 1632 (C⚌N), 1699 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.79 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ph-H), 7.75–7.74 (m, 3H, Ph-H & NH), 7.54 (d, J = 4.4 Hz, 2H, Ph-H), 7.16 (d, J = 8.0 Hz, 2H, Ph-H), 7.09 (s, 1H, thiazole-H), 4.33 (s, 2H, CH2), 2.85–2.83 (m, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.35–1.33 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.8 (C⚌O), 165.5 (C⚌N), 148.8 (C), 143.0 (C), 134.3 (C), 131.8 (CH), 130.3 (C), 130.0 (C), 129.2 (CH), 128.0 (CH), 119.6 (CH), 102.9 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.3.4 N-[4-(piperidine-1-sulfonyl)phenyl]-2-(4-(pyren-1-yl)thiazol-2-ylamino)acetamide (7d)
Dense yellow crystals; yield 68%; mp 165–167 °C; FT-IR = 3345 (NH), 3268 (NH), 3046 (CH arom.), 2848 (CH aliph.), 1691 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 11.02 (s, 1H, NH), 8.83 (d, J = 4.8 Hz, 1H, Pyr-H), 8.29–8.22 (m, 4H, Pyr-H), 8.20–8.18 (m, 3H, Pyr-H), 8.08 (d, J = 4.8 Hz, 1H, Pyr-H), 7.88 (d, J = 8.4 Hz, 2H, Ph-H), 7.69 (d, J = 8.4 Hz, 2H, Ph-H), 7.31 (s, 1H, NH), 6.98 (s, 1H, thiazole-H), 4.37 (s, 2H, CH2), 2.85–2.83 (m, 4H, 2CH2), 1.52–1.50 (m, 4H, 2CH2), 1.32–1.30 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.7 (C⚌O), 165.9 (C⚌N), 153.5 (C), 152.4 (C), 150.3 (C), 143.0 (C), 131.4 (C), 131.2 (C), 130.8 (C), 130.7 (C), 130.2 (C), 129.9 (CH), 129.2 (CH), 127.8 (CH), 127.7 (CH), 127.2 (C), 126.8 (CH), 126.1 (CH), 125.7 (CH), 125.3 (CH), 120.1 (CH), 119.6 (CH), 113.1 (CH), 106.3 (CH), 47.0 (CH2), 44.0 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2.1.3.5 2-[4-(9H-fluoren-2-yl)thiazol-2-ylamino]-N-[4-(piperidine-1-sulfonyl)phenyl]acetamide (7e)
Pale yellow crystals; yield 84%; mp 195–197 °C; FT-IR = 3312 (NH), 3119 (NH), 3061 (CH arom.), 2940 (CH aliph.), 1693 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.76 (s, 1H, NH), 10.62 (s, 1H, NH), 8.03 (s, 1H, Flu-H), 7.88 (d, J = 8.0 Hz, 2H, Ph-H), 7.83 (d, J = 4.0 Hz, 1H, Flu-H), 7.72 (d, J = 8.0 Hz, 2H, Ph-H), 7.69 (d, J = 4.0 Hz, 1H, Flu-H), 7.60–7.58 (m, 1H, Flu-H), 7.38 (d, J = 5.0 Hz, 1H, Flu-H), 7.33–7.31 (m, 1H, Flu-H), 7.09 (s, 1H, Flu-H), 7.06 (s, 1H, thiazole-H), 4.33 (s, 2H, CH2), 3.95 (s, 2H, CH2), 2.86–2.84 (m, 4H, 2CH2), 1.55–1.53 (m, 4H, 2CH2), 1.35–1.33 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.5 (C⚌O), 165.8 (C⚌N), 150.5 (C), 143.8 (C), 143.7 (C), 142.9 (C), 141.4 (C), 140.6 (C), 134.0 (C), 130.3 (C), 129.2 (CH), 127.2 (CH), 127.1 (CH), 125.8 (CH), 125.6 (CH), 124.9 (CH), 122.7 (CH), 120.4 (CH), 119.6 (CH), 101.7 (CH), 47.0 (CH2), 44.0 (CH2), 36.9 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2.1.3.6 2-[4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-ylamino]-N-[4-(piperidine-1-sulfonyl)phenyl]-acetamide (7f)
Pale yellow plates; yield 86%; mp 115–117 °C; FT-IR = 3292 (NH), 3118 (NH), 3068 (CH arom.), 2943 (CH aliph.), 1695 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.84 (s, 1H, NH), 7.86–7.83 (m, 3H, 2 × Ph-H & Flu-H), 7.71–7.65 (m, 4H, 2 × Ph-H & 2 × Flu-H), 7.48 (s, 1H, NH), 7.35 (d, J = 4.0 Hz, 1H, Flu-H), 7.19 (s, 1H, Flu-H), 6.78 (s, 1H, thiazole-H), 4.34 (s, 2H, CH2), 4.01 (s, 2H, CH2), 2.86–2.83 (m, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.36–1.34 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.9 (C⚌O), 165.8 (C⚌N), 148.6 (C), 146.6 (C), 146.1 (C), 143.0 (C), 139.1 (C), 136.9 (C), 133.5 (C), 131.9 (C), 131.5 (C), 130.3 (C), 129.2 (CH), 128.5 (CH), 126.9 (CH), 125.4 (CH), 125.0 (CH), 122.8 (CH), 119.6 (CH), 105.7 (CH), 47.0 (CH2), 44.0 (CH2), 36.9 (CH2), 25.1 (CH2), 23.3 (CH2) ppm.
2.1.3.7 N-[4-(morpholine-4-sulfonyl)phenyl]-2-(thiazol-2-ylamino)acetamide (7g)
Pale yellow crystals; yield 94%; mp 120–122 °C; FT-IR = 3332 (NH), 3210 (NH), 3055 (CH arom.), 2859 (CH aliph.), 1701 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 11.18 (s, 1H, NH), 7.91 (d, J = 8.0 Hz, 2H, Ph-H), 7.70 (d, J = 8.0 Hz, 2H, Ph-H), 7.35–7.33 (m, 2H, thiazole-H), 5.05 (s, 1H, NH), 4.38 (s, 2H, CH2), 3.61 (t, J = 4.0 Hz, 4H, 2CH2), 2.83 (t, J = 4.0 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 165.9 (C⚌O), 165.8 (C⚌N), 143.4 (C), 129.8 (C), 129.4 (CH), 129.3 (CH), 119.6 (CH), 112.8 (CH), 67.0 (CH2), 46.3 (CH2), 43.9 (CH2) ppm.
2.1.3.8 N-[4-(morpholine-4-sulfonyl)phenyl]-2-(4-phenylthiazol-2-ylamino)acetamide (7h)
Pale yellow plates; yield 69%; mp 110–113 °C; FT-IR = 3328 (NH), 3114 (NH), 3068 (CH arom.), 2859 (CH aliph.), 1699 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.80 (s, 1H, NH), 10.66 (s, 1H, NH), 7.88 (d, J = 8.4 Hz, 2H, Ph-H), 7.79 (d, J = 8.4 Hz, 2H, Ph-H), 7.73 (d, J = 8.5 Hz, 2H, Ph-H), 7.37–7.33 (m, 2H, Ph-H), 7.08–7.06 (m, 1H, Ph-H), 6.99 (s, 1H, thiazole-H), 4.34 (s, 2H, CH2), 3.61 (t, J = 5.2 Hz, 4H, 2CH2), 2.83 (t, J = 5.2 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.8 (C⚌O), 165.9 (C⚌N), 150.3 (C), 143.8 (C), 143.3 (C), 135.3 (C), 129.5 (CH), 128.9 (CH), 127.6 (CH), 126.0 (CH), 119.6 (CH), 101.9 (CH), 65.7 (CH2), 46.7 (CH2), 44.0 (CH2) ppm.
2.1.3.9 N-[4-(4-bromophenyl)thiazol-2-yl]-2-[4-(morpholine-4-sulfonyl)phenylamino]acetamide (7i)
Pale yellow powder; yield 65%; mp 130–132 °C; FT-IR = 3328 (NH), 3112 (NH), 3010 (CH arom.), 2860 (CH aliph.), 1700 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 10.81 (s, 1H, NH), 7.87 (d, J = 8.8 Hz, 2H, Ph-H), 7.75–7.71 (m, 3H, Ph-H + thiazole-H), 7.54 (d, J = 8.0 Hz, 2H, Ph-H), 7.09 (d, J = 8.8 Hz, 2H, Ph-H), 6.13 (s, 1H, NH), 4.33 (s, 2H, CH2), 3.62 (t, J = 4.2 Hz, 4H, 2CH2), 2.83 (t, J = 4.2 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.8 (C⚌O), 165.9 (C⚌N), 149.0 (C), 143.3 (C), 134.5 (C), 131.8 (CH), 130.0 (C), 129.5 (CH), 128.0 (CH), 120.5 (C), 119.6 (CH), 102.8 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2) ppm.
2.1.3.10 N-(4-(morpholinosulfonyl)phenyl)-2-(4-(pyren-1-yl)thiazol-2-ylamino)acetamide (7j)
Yellow crystals; yield 51%; mp 170–173 °C; FT-IR = 3364 (NH), 3200 (NH), 3045 (CH arom.), 2851 (CH aliph.), 1689 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 8.86 (d, J = 8.4 Hz, 1H, Pyr-H), 8.29–8.22 (m, 4H, Pyr-H), 8.20–8.18 (m, 2H, Pyr-H), 8.08–8.04 (m, 2H, Pyr-H), 7.92 (d, J = 8.8 Hz, 2H, Ph-H), 7.70 (d, J = 8.8 Hz, 2H, Ph-H), 6.95 (s, 1H, thiazole-H), 6.70 (s, 1H, NH), 6.16 (s, 1H, NH), 4.38 (s, 2H, CH2), 3.61 (t, J = 4.8 Hz, 4H, 2CH2), 2.76 (t, J = 4.8 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.6 (C⚌O), 166.0 (C⚌N), 153.8 (C), 150.7 (C), 143.1 (C), 131.4 (C), 131.2 (C), 130.9 (C), 130.6 (C), 130.2 (CH), 129.5 (CH), 127.8 (CH), 127.7 (CH), 127.5 (C), 126.7 (CH), 126.2 (CH), 125.6 (CH), 125.4 (CH), 124.7 (C), 124.5 (C), 119.4 (CH), 113.2 (CH), 106.3 (CH), 65.7 (CH2), 45.9 (CH2), 44.0 (CH2) ppm.
2.1.3.11 2-[4-(9H-fluoren-2-yl)thiazol-2-ylamino]-N-[4-(morpholine-4-sulfonyl)phenyl]acetamide (7k)
Pale yellow crystals; yield 77%; mp 185–187 °C; FT-IR = 3319 (NH), 3118 (NH), 3056 (CH arom.), 2917 (CH aliph.), 1695 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 11.10 (s, 1H, NH), 8.16 (s, 1H, Flu-H), 8.04–8.01 (m, 1H, Flu-H), 7.91 (d, J = 8.4 Hz, 1H, Flu-H), 7.87 (d, J = 8.8 Hz, 2H, Ph-H), 7.73 (d, J = 8.8 Hz, 2H, Ph-H), 7.69 (s, 1H, thiazole-H), 7.58–7.40 (m, 2H, Flu-H), 7.37–7.30 (m, 2H, Flu-H), 7.07 (s, 1H, NH), 4.37 (s, 2H, CH2), 3.93 (s, 2H, CH2), 3.62 (t, J = 4.0 Hz, 4H, 2CH2), 2.83 (t, J = 4.0 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.5 (C⚌O), 165.9 (C⚌N), 150.6 (C), 143.7 (C), 143.4 (C), 141.4 (C), 140.6 (C), 134.3 (C), 129.4 (CH), 129.0 (C), 127.2 (CH), 127.1 (CH), 125.5 (CH), 124.9 (CH), 122.7 (CH), 120.4 (CH), 119.6 (CH), 119.4 (CH), 101.6 (CH), 65.7 (CH2), 46.3 (CH2), 44.0 (CH2), 36.8 (CH2) ppm.
2.1.3.12 2-[4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-ylamino]-N-[4-(morpholine-4-sulfonyl)phenyl]-acetamide (7l)
Pale yellow crystals; yield 94%; mp 155–157 °C; FT-IR = 3329 (NH), 3111 (NH), 3056 (CH arom.), 2976 (CH aliph.), 1701 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 11.44 (s, 1H, NH), 8.25 (s, 1H, NH), 7.95 (d, J = 9.0 Hz, 2H, Ph-H), 7.70 (d, J = 8.4 Hz, 1H, Flu-H), 7.63–7.61 (m, 3H, Flu-H & 2 × Ph-H), 7.50 (d, J = 8.4 Hz, 1H, Flu-H), 7.34 (s, 1H, Flu-H), 7.22 (s, 1H, Flu-H), 6.76 (s, 1H, thiazole-H), 4.42 (s, 2H, CH2), 3.98 (s, 2H, CH2), 3.61 (t, J = 4.0 Hz, 4H, 2CH2), 2.83 (t, J = 4.0 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.9 (C⚌O), 166.0 (C⚌N), 148.6 (C), 146.6 (C), 146.1 (C), 143.5 (C), 139.1 (C), 136.9 (C), 133.5 (C), 131.9 (C), 131.4 (C), 129.3 (CH), 128.9 (CH), 128.5 (CH), 126.9 (CH), 125.3 (CH), 124.9 (CH), 119.6 (CH), 105.7 (CH), 65.7 (CH2), 45.9 (CH2), 43.9 (CH2), 36.9 (CH2) ppm.
2.1.4 General procedure for synthesis of N-(4-substituted-thiazol-2-yl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamides 8a-f and 2-(4-(morpholinosulfonyl)phenylamino)-N-(4-substituted-thiazol-2-yl)acetamides 8g-l
In a round bottom flask, 2-chloro-N-(4-substituted-thiazol-2-yl)acetamide derivatives 4a-f (0.001 mol), sulfonamide 5a or 5b (0.001 mol) and triethylamine (0.1 mL) in absolute ethanol (20 mL) was refluxed for 5–9 h. The reaction mixture was cooled to room temperature, the obtained solid was filtered, washed with cold ethanol, dried and crystallized from ethanol to give the titled products 8a-f and 8g-l.
2.1.4.1 2-[4-(Piperidine-1-sulfonyl)phenylamino]-N-thiazol-2-yl-acetamide (8a)
Pale yellow crystals; yield 75%; mp 160–162 °C; FT-IR = 3362 (NH), 3215 (NH), 3048 (CH arom.), 2838 (CH aliph.), 1660 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 11.12 (s, 1H, NH), 7.83 (d, J = 8.4 Hz, 2H, Ph-H), 7.71 (d, J = 8.4 Hz, 2H, Ph-H), 7.43 (d, J = 8.8 Hz, 1H, thiazole-H), 7.01 (d, J = 8.8 HZ, 1H, thiazole-H), 4.42 (s, 2H, CH2), 2.84–2.82 (m, 4H, 2CH2), 1.54–1.52 (m, 4H, 2CH2), 1.33–1.31 (m, H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 169.8 (C⚌O), 165.7 (C⚌N), 142.6 (C), 131.4 (CH), 130.2 (C), 129.5 (CH), 119.2 (CH), 106.6 (CH), 47.1 (CH2), 44.0 (CH2), 25.1 (CH2), 23.2 (CH2) ppm.
2.1.4.2 N-(4-phenylthiazol-2-yl)-2-[4-(piperidine-1-sulfonyl)phenylamino]acetamide (8b)
Pale yellow crystals; yield 92%; mp 115–117 °C; FT-IR = 3362 (NH), 3180 (NH), 3063 (CH arom.), 2948 (CH aliph.), 1698 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.71 (s, 1H, NH), 7.91 (d, J = 6.5 Hz, 2H, Ph-H), 7.70 (s, 1H, thiazole-H), 7.69–7.65 (m, 1H, Ph-H), 7.44–7.42 (m, 3H, Ph-H & NH), 7.33 (d, J = 7.6 Hz, 2H, Ph-H), 6.67 (d, J = 7.6 Hz, 2H, Ph-H), 4.44 (s, 2H, CH2), 2.78–2.75 (m, 4H, 2CH2), 1.52–1.50 (m, 4H, 2CH2), 1.34–1.31 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.2 (C⚌O), 165.0 (C⚌N), 157.9 (C), 153.3 (C), 149.5 (C), 134.5 (C), 129.8 (CH), 129.2 (CH), 128.3 (CH), 126.1 (CH), 113.2 (CH), 109.0 (CH), 47.0 (CH2), 42.7 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.4.3 N-[4-(4-bromophenyl)thiazol-2-yl]-2-[4-(piperidine-1-sulfonyl)phenylamino]acetamide (8c)
Pale yellow crystals; yield 93%; mp 120–123 °C; FT-IR = 3361 (NH), 3183 (NH), 3048 (CH arom.), 2952, 2837 (CH aliph.), 1647 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.22 (s, 1H, NH), 7.85 (d, J = 9.2 Hz, 2H, Ph-H), 7.75 (d, J = 7.6 Hz, 2H, Ph-H), 7.62 (s, 1H, thiazole-H), 7.31 (d, J = 9.2 Hz, 2H, Ph-H), 7.08 (s, 1H, NH), 6.65 (d, J = 7.6 Hz, 2H, Ph-H), 4.44 (s, 2H, CH2), 2.77–2.75 (m, 4H, 2CH2), 1.53–1.50 (m, 4H, 2CH2), 1.33–1.31 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.9 (C⚌O), 165.7 (C⚌N), 158.1 (C), 153.5 (C), 148.3 (C), 134.2 (C), 133.7 (C), 132.1 (CH), 129.8 (CH), 128.2 (CH), 113.1 (CH), 102.9 (CH), 47.0 (CH2), 42.7 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.4.4 2-(4-(Piperidin-1-ylsulfonyl)phenylamino)-N-(4-(pyren-1-yl)thiazol-2-yl)acetamide (8d)
Pale yellow crystals; yield 74%; mp 155–156 °C; FT-IR = 3362 (NH), 3176 (NH), 3043 (CH arom.), 2947 (CH aliph.), 1698 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.90 (s, 1H, NH), 8.74–8.71 (m, 2H, Pyr-H), 8.32–8.30 (m, 4H, Pyr-H), 8.21–8.19 (m, 2H, Pyr-H), 8.08 (d, J = 4.4 Hz, 1H, Pyr-H), 7.66 (s, 1H, thiazole-H), 7.34 (d, J = 8.0 Hz, 2H, Ph-H), 7.09 (s, 1H, NH), 6.70 (d, J = 8.0 Hz, 2H, Ph-H), 4.52 (s, 2H, CH2), 2.77–2.75 (m, 4H, 2CH2), 1.49–1.47 (m, 4H, 2CH2), 1.28–1.26 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 169.8 (C⚌O), 165.2 (C⚌N), 157.8 (C), 153.4 (C), 149.9 (C), 131.5 (C), 131.4 (C), 131.3 (C), 131.1 (C), 130.8 (C), 130.7 (C), 130.3 (C), 129.9 (CH), 128.3 (CH), 128.0 (CH), 127.8 (CH), 126.9 (CH), 125.9 (CH), 125.4 (CH), 124.7 (CH), 124.4 (CH), 120.5 (CH), 113.2 (CH), 106.7 (CH), 47.0 (CH2), 42.8 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.4.5 N-[4-(9H-fluoren-2-yl)thiazol-2-yl]-2-[4-(piperidine-1-sulfonyl)phenylamino]acetamide (8e)
Pale yellow crystals; yield 78%; mp 155–157 °C; FT-IR = 3360 (NH), 3185 (NH), 3064 (CH arom.), 2837 (CH aliph.), 1647 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.75 (s, 1H, NH), 8.13 (s, 1H, NH), 8.02 (s, 1H, Flu-H), 7.96–7.91 (m, 3H, Flu-H), 7.73 (d, J = 4.8 Hz, 1H, Flu-H), 7.61 (s, 1H, thiazole-H), 7.42–7.38 (m, 2H, Flu-H), 7.34 (d, J = 9.2 Hz, 2H, Ph-H), 6.67 (d, J = 9.2 Hz, 2H, Ph-H), 4.47 (s, 2H, CH2), 3.99 (s, 2H, CH2), 2.78–2.76 (m, 4H, 2CH2), 1.53–1.51 (m, 4H, 2CH2), 1.33–1.31 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 165.6 (C⚌O), 158.1 (C⚌N), 157.7 (C), 153.5 (C), 149.8 (C), 144.0 (C), 143.8 (C), 141.3 (C), 141.2 (C), 133.2 (C), 129.8 (CH), 127.3 (CH), 125.6 (CH), 125.1 (CH), 122.9 (CH), 120.7 (CH), 120.6 (CH), 120.3 (CH), 113.1 (CH), 108.7 (CH), 47.0 (CH2), 42.8 (CH2), 36.9 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.4.6 N-[4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl]-2-[4-(piperidine-1-sulfonyl)phenylamino]-acetamide (8f)
Pale yellow crystals; yield 84%; mp; 125–127 °C; FT-IR = 3362 (NH), 3113 (NH), 3065 (CH arom.), 2947 (CH aliph.), 1686 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.81 (s, 1H, NH), 7.71 (s, 1H, Flu-H), 7.65 (s, 1H, Flu-H), 7.50 (s, 1H, Flu-H), 7.40 (s, 1H, thiazole-H), 7.34–7.32 (m, 4H, 2 × Ph-H & 2 × Flu-H), 6.66 (d, J = 9.2 Hz, 2H, Ph-H), 6.13 (s, 1H, NH), 4.48 (s, 2H, CH2), 4.02 (s, 2H, CH2), 2.77–2.75 (m, 4H, 2CH2), 1.53–1.50 (m, 4H, 2CH2), 1.33–1.31 (m, 2H, CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 169.1 (C⚌O), 165.8 (C⚌N), 158.0 (C), 153.5 (C), 147.8 (C), 146.8 (C), 146.2 (C), 138.8 (C), 137.0 (C), 132.5 (C), 132.1 (C), 131.6 (C), 129.8 (CH), 129.0 (CH), 127.1 (CH), 125.5 (CH), 124.6 (CH), 120.3 (CH), 113.1 (CH), 105.7 (CH), 47.0 (CH2), 42.7 (CH2), 36.9 (CH2), 25.1 (CH2), 23.4 (CH2) ppm.
2.1.4.7 2-[4-(Morpholine-4-sulfonyl)phenylamino]-N-thiazol-2-yl-acetamide (8g)
Pale yellow crystals; yield 76%; mp 210–212 °C; FT-IR = 3364 (NH), 3198 (NH), 3038 (CH arom.), 2984 (CH aliph.), 1643 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.98 (s, 1H, NH), 7.36 (d, J = 8.0 Hz, 2H, Ph-H), 6.66 (d, J = 8.0 Hz, 2H, Ph-H), 6.13–6.11 (m, 2H, thiazole-H), 5.00 (s, 1H, NH), 4.42 (s, 2H, CH2), 3.61 (t, J = 4.0 Hz, 4H, 2CH2), 2.76 (t, J = 4.0 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 167.2 (C⚌O), 165.3 (C⚌N), 153.8 (C), 130.1 (CH), 119.1 (CH), 113.1 (CH), 109.0 (CH), 65.7 (CH2), 46.3 (CH2), 42.4 (CH2) ppm.
2.1.4.8 2-[4-(Morpholine-4-sulfonyl)phenylamino]-N-(4-phenylthiazol-2-yl)acetamide (8h)
Pale yellow crystals; yield 92%; mp 135–136 °C; FT-IR = 3364 (NH), 3108 (NH), 3060 (CH arom.), 2983 (CH aliph.), 1682 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.66 (s, 1H, NH), 7.90 (d, J = 8.4 Hz, 2H, Ph-H), 7.68 (s, 1H, thiazole-H), 7.45–7.42 (m, 2H, Ph-H), 7.37–7.32 (m, 3H, Ph-H), 6.68 (d, J = 8.0 Hz, 2H, Ph-H), 6.13 (s, 1H, NH), 4.40 (s, 2H, CH2), 3.60 (t, J = 4.8 Hz, 4H, 2CH2), 2.76 (t, J = 4.8 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.2 (C⚌O), 165.6 (C⚌N), 157.9 (C), 153.8 (C), 149.5 (C), 134.5 (C), 130.2 (CH), 129.2 (CH), 128.3 (CH), 126.1 (CH), 119.1 (CH), 113.1 (CH), 109.0 (CH), 64.8 (CH2), 45.2 (CH2), 42.7 (CH2) ppm.
2.1.4.9 N-[4-(4-bromophenyl)thiazol-2-yl]-2-[4-(morpholine-4-sulfonyl)-phenylamino]acetamide (8i)
Pale yellow crystals; yield 91%; mp 135–137 °C; FT-IR = 3364 (NH), 3209 (NH), 3076 (CH arom.), 2986 (CH aliph.), 1650 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.44 (s, 1H, NH), 7.84 (d, J = 8.0 Hz, 2H, Ph-H), 7.74 (s, 1H, thiazole-H), 7.62 (d, J = 8.0 Hz, 2H, Ph-H), 7.34 (d, J = 8.0 Hz, 2H, Ph-H), 6.68 (d, J = 8.0 Hz, 2H, Ph-H), 6.12 (s, 1H, NH), 4.43 (s, 2H, CH2), 3.66 (t, J = 4.4 Hz, 4H, 2CH2), 2.75 (t, J = 4.4 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 168.8 (C⚌O), 165.6 (C⚌N), 158.1 (C), 153.8 (C), 149.1 (C), 134.5 (C), 132.1 (CH), 131.8 (C), 130.2 (CH), 128.1 (CH), 113.2 (CH), 109.9 (CH), 65.7 (CH2), 46.3 (CH2), 42.4 (CH2) ppm.
2.1.4.10 2-[4-(Morpholine-4-sulfonyl)phenylamino]-N-(4-pyren-1-yl-thiazol-2-yl)acetamide (8j)
Pale yellow crystals; yield 95%; mp 190–192 °C; FT-IR = 3364 (NH), 3180 (NH), 3070 (CH arom.), 2984 (CH aliph.), 1701 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.70 (s, 1H, NH), 8.25 (d, J = 4.4 Hz, 1H, Pyr-H), 7.71–7.67 (m, 3H, Pyr-H), 7.65–7.62 (m, 2H, Pyr-H), 7.51 (d, J = 4.4 Hz, 1H, Pyr-H), 7.35 (d, J = 8.4 Hz, 2H, Ph-H), 7.27–7.20 (m, 2H, Pyr-H), 6.68 (d, J = 8.4 Hz, 2H, Ph-H), 6.14 (s, 1H, thiazole-H), 4.45 (s, 1H, NH), 4.02 (s, 2H, CH2), 3.61 (t, J = 4.8 Hz, 4H, 2CH2), 2.76 (t, J = 4.8 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 165.7 (C⚌O), 158.0 (C⚌N), 153.8 (C), 148.7 (C), 147.8 (C), 146.8 (C), 146.2 (C), 138.8 (C), 137.0 (C), 132.5 (C), 132.1 (C), 131.6 (C), 130.1 (CH), 129.0 (CH), 127.1 (CH), 126.9 (CH), 125.5 (CH), 125.3 (CH), 124.6 (CH), 119.0 (CH), 113.3 (CH), 113.1 (CH), 105.4 (CH), 65.7 (CH2), 45.9 (CH2), 42.8 (CH2) ppm.
2.1.4.11 N-[4-(9H-fluoren-2-yl)thiazol-2-yl]-2-[4-(morpholine-4-sulfonyl)phenylamino]acetamide (8k)
Pale yellow crystals; yield 98%; mp 185–187 °C; FT-IR = 3365 (NH), 3200 (NH), 3065 (CH arom.), 2985 (CH aliph.), 1651 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 12.76 (s, 1H, NH), 8.16–8.12 (m, 2H, Flu-H), 8.08–8.00 (m, 1H, Flu-H), 7.94–7.91 (m, 1H, Flu-H), 7.89–7.85 (m, 2H, Flu-H), 7.72 (s, 1H, thiazole-H), 7.59 (d, J = 7.6 Hz, 1H, Flu-H), 7.35 (d, J = 8.0 Hz, 2H, Ph-H), 6.69 (d, J = 8.0 Hz, 2H, Ph-H), 6.20 (s, 1H, NH), 4.48 (s, 2H, CH2), 3.97 (s, 2H, CH2), 3.60 (t, J = 4.0 Hz, 4H, 2CH2), 2.75 (t, J = 4.0 Hz, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 165.7 (C⚌O), 158.0 (C⚌N), 153.6 (C), 148.1 (C), 147.6 (C), 146.6 (C), 146.2 (C), 138.6 (C), 137.0 (CH), 132.1 (C), 130.2 (CH), 129.1 (CH), 127.0 (CH), 126.8 (CH), 125.5 (CH), 124.8 (CH), 119.1 (CH), 113.2 (CH), 105.4 (CH), 66.0 (CH2), 46.0 (CH2), 42.3 (CH2), 36.9 (CH2) ppm.
2.1.4.12 N-[4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl]-2-[4-(morpholine-4-sulfonyl)phenylamino]-acetamide (8l)
Pale yellow crystals; yield 84%; mp 120–122 °C; FT-IR = 3364 (NH), 3113 (NH), 3066 (CH arom.), 2982 (CH aliph.), 1690 (C⚌O) cm−1; 1H NMR (DMSO‑d6, 400 MHz): δ = 7.72 (s, 1H, Flu-H), 7.66 (s, 1H, Flu-H), 7.50 (s, 1H, Flu-H), 7.40 (s, 1H, thiazole-H), 7.36–7.34 (m, 4H, 2 × Flu-H & 2 × Ph-H), 7.19 (s, 1H, NH), 6.68 (d, J = 8.0 Hz, 2H, Ph-H), 5.83 (s, 1H, NH), 4.44 (s, 2H, CH2), 4.03 (s, 2H, CH2), 3.61 (m, 4H, 2CH2), 2.75 (m, 4H, 2CH2) ppm; 13C NMR (DMSO‑d6, 100 MHz): δ = 165.8 (C⚌O), 158.1 (C⚌N), 158.0 (C), 153.8 (C), 147.8 (C), 146.8 (C), 146.3 (C), 138.8 (C), 137.0 (C), 132.5 (C), 132.1 (C), 131.6 (C), 130.1 (CH), 129.0 (CH), 127.1 (CH), 125.5 (CH), 124.6 (CH), 119.1 (CH), 113.2 (CH), 105.8 (CH), 65.7 (CH2), 46.3 (CH2), 42.7 (CH2), 36.9 (CH2) ppm.
2.2 Biological evaluation
2.2.1 Antimicrobial screening
2.2.1.1 Used microorganisms
All microbial strains were kindly provided from the department of Medical Microbiology and Immunology faculty of Medicine Assiut University, these clinical isolates were obtained from clinical cases of infections admitted to Assiut University hospital as urinary tract infections, corneal ulcers, bacterial and fungal pneumonia, otomycosis, oral thrush and wound infections. The clinical isolates were proved by using the VITEK 2 automated microbiology system (BioMérieux). The clinical isolates used were multidrug resistant strains particularly for trimethoprim/sulfamethoxazole (25 µg) (oxoid CT0052B), they included Gram positive bacteria as Staphylococcus aureus (S. aureus), Methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae (S. pneumoniae), and Gram negative bacteria as Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), Pseudomonas aeruginosa (P. aeruginosa), and Acinetobacter baumannii (A. baumannii). The fungal strains that were tested are Aspergillus flavus (A. flavus), A. niger (A. niger) and Candida albicans (C. albicans).
2.2.1.2 Initial evaluation of the sulfonamide derivatives antibacterial and antifungal activity
The antimicrobial activity of the sulfonamide derivatives was initially evaluated by agar well diffusion assay (Ginovyan et al., 2015). Mueller Hinton agar (CM0337) was poured into Petri dishes at 50–60 °C and left to solidify for 15 min. Subsequently, overnight microbial suspensions of tested strains was adjusted to turbidity of 0.5 McFarland Standard, which equals to 1–2 × 108 CFU/mL for bacteria and 1–5 × 106 for fungi. The microbial inoculums were then diluted in 1:100 ratio in case of bacteria and 1:10 ratio in case of fungi in order to get 1-5 × 105 CFU/mL a sterile cotton swab was dipped into the adjusted microbial suspension and the Mueller Hinton agar plates were inoculated by evenly streaking cotton swab over the agar medium. Then wells with a diameter of 0.5 cm were cut in the medium with a sterile cork borer. Stock solutions of the sulfonamide derivatives were diluted in DMSO 1% to get 500 µg/mL concentrations. The tested sulfonamide derivatives and controls (50 μL) were dispensed into the wells. The plates were incubated for 24 h at 37 °C for bacteria and C. albicans while at 25 °C for A. falvus and A. niger. The diameters of zones of inhibition (ZOI) around the wells were measured in mm. Following control agents were used: positive control agents – vancomycin (50 µg/mL) for Gram positive bacteria, gentamicin (10 µg/mL) (for Gram negative bacteria) and fluconazole 25 µg/mL for fungi and negativen control agent is 1% DMSO.
2.2.1.3 Determination of MIC values for the most active sulfonamide derivatives
Determination of Minimum inhibitory concentrations (MIC) of sulfonamide derivatives was done using broth microdilution method (Wiegand et al., 2008). The procedure involved preparation of two-fold dilutions of the sulfonamide derivatives ranging from (500–7.8 µg/mL) in sterile Mueller Hinton broth inside the wells of 96-well microplate (Sarstedt, Germany). The inoculums of test strains prepared from fresh overnight cultures were adjusted to 0.5 McFarland standards, which equals to 1–2 × 108 CFU/mL for bacteria (CLSI, 2012). The highest dilution of samples (sulfonamide derivatives) without visible growth after 24 h incubation at 37 °C was considered as MIC. For this assay the positive control agents were vancomycin (range: 0.7–50 µg/mL), gentamicin (range: 0.15–10 µg/mL) and the negative control was 1% DMSO.
For proper determination of the MIC end point resazurin dye has been used (Khalifa et al., 2013). A stock solution of resazurin sodium salt powder (Titan Biotech) was prepared at 0.02% (w/v) in distilled water, sterilized by filtration through a 0–2 µm filter into a sterile light protected container then stored protected from light at 4 °C for up to 1 week, or at −20% for long term use, then 10–15% resazurin solution of the total volume in wells was added to each well and incubation for 1–4 h at 37 °C was done. A change in color from blue to pink indicates the growth of bacteria, and MIC was defined as the lowest concentration of the drug that prevented this change in color.
2.2.1.4 Data processing
All experiments were independently repeated three times. Obtained data were processed; standard deviations were calculated using GraphPad Prism 5.03 (GraphPad Software, Inc.; USA) software.
2.2.1.5 Media and reagents
Muller Hinton agar oxoid code: CM0337, Muller Hinton broth oxoid code: CM 0405, Mannitol salt agar oxoid code: CM 0151, Columbia agar oxoid code: CM 0331, and Orsab oxoid code CM 1008.
2.2.2 In vitro anticancer screening
2.2.2.1 Cell culture
WI-38 normal lung fibroblast cells, A549 lung cancer cells, and MDA-MB-231 breast cancer cells were obtained from VACSERA - Cell Culture Unit, Cairo, Egypt. The cell lines were originally obtained from the American Tissue Culture Collection (ATCC). WI-38, A549, and MDA-MB-231 cell lines were cultured in RPMI-1640 medium supplemented with 10% inactivate fetal bovine serum (FBS) and 1% penicilin/streptomycin.
2.2.2.2 Cell viability assay
WI-38, A549, and MDA-MB-231 cells were seeded into 96-well plates (at a density of 5000 cells/well). On the following day, cells were treated with different concentrations (0, 1, 10, 31.25, 62.5, 125, 250, 500 µg/mL) of 24 sulfonamide derivatives in fresh medium and incubated for another 24 hrs. Cell viability was then assessed using the MTT assay (Sigma Aldrich, St. Louis, MO, USA), and the absorbance was read at 570 nM using an ELISA microplate reader (Molecular Devices, Downingtown, PA, USA).
2.2.2.3 FACS analysis
To uncover the mechanism of cell death for 8d, 8e, and 8f sulfonamide derivatives on WI-38, A549, and MDA-MB-231 cells; Annexin v and propedium Iodide (PI) were used. In brief, WI-38, A549, MDA-MB-231 cells were cultured in 10 tissue culture dish with initial number 4 × 105 cell/Ml in RPMI growth media and treated with 8d, 8e, and 8f sulfonamide derivatives as the following; (0.0 and 1000 µg/mL for each) for WI-38, (36, 16, and 22) for A549, and (12, 72, 72) for MDA-MB-231 24 h and incubated for 24 h and then the cells were washed and trypsnized and suspended in 50 µL 1X Annexin v binding buffer followed by adding 5 µL FITC Annexin V and incubated for 15 min at room temperature then 5 µL of PI were added to each tube. Finally, 400 µL of 1X Annexin v binding buffer were added to each tube and analyzed using Becton Dickinson FACS Caliber.
2.3 Molecular modeling and docking simulation
Molecular modeling and docking simulation studies were carried out at the Department of Medicinal Chemistry, Faculty of Pharmacy, Assiut University, Assiut, Egypt.
2.3.1 Software and hardware
All the molecular modeling studies and docking simulation studies were performed using Molecular Operating Environment (MOE®) version 2019.01, Chemical Computing Group (CCG) Inc., Montreal, Canada [Molecular Operating Enviroment (MOE), Version, Chemical Computing group Inc., Montreal, Quebec, Canada, 2019. http://www.chemcomp.com.]. The computational software operated under “Windows 8.1 operating system” installed on an Intel (R) Xeon (R) CPU E5-1650 3.20 GHz processor, 16 GB memory. All the interaction energies and different calculations were automatically calculated.
2.3.2 Target compounds optimization
The target compounds were constructed into a 3D model using the builder interface of the MOE program. After checking their structures and the formal charges on atoms by 2D depiction, the following steps were carried out. The target compounds were subjected to a conformational search. All conformers were subjected to energy minimization, all the minimizations were performed with MOE until a RMSD gradient of 0.0001 Kcal/mole and RMS distance of 0.1 Å with MMFF94X forcefield and the partial charges were automatically calculated. The obtained data base was then saved as MDB file to be used in the docking calculations.
2.3.3 Optimization of the receptor active site
X-ray Crystal Structure of human dihydrofolate reductase (DHRF) complexed with folate (PDB ID: 1DRF) was obtained from the Protein Data Bank [https://www.rcsb.org] at 2.0°A resolution. The DHRF enzyme was prepared for docking by using QUICKPREP protocol embedded in the MOE software. Hydrogen atoms were added to the system with their standard geometry. The atoms connection and type were checked for any errors with automatic correction. Selection of the receptor and its atoms potential were fixed. MOE Alpha Site Finder was used for the active site search in the enzyme structure using all default items. Dummy atoms were created from the obtained alpha DHFR active sites. Redocking of the co-crystallized Ligand (Folate) was done to verify the docking methodology to be used in the oncoming synthesized compounds docking.
2.3.4 Docking of the conformation database of the target compounds was done using MOE-Dock protocol
The following methodology was generally applied: The active site file was loaded, and the Dock tool was initiated. The program specifications were adjusted to: the dummy atoms as the docking site, alpha triangle as the placement methodology with timeout (300 Sec) and number of Return poses = 1000. The London dG as scoring methodology determines the free energy of binding of the ligand from a given pose. A number of 50 cycles of calculation has been carried out to determine the best poses of the docked molecules. Rigid receptor refinement with a final gradient of 0.01 with a maximum of 500 Iterations was used afterwards and the GBVI/WBA dG forcefield based rescoring was applied to retain the best 30 poses. The MDB file of the ligand to be docked was loaded and Dock calculations were run automatically, the produced dock file was created with different poses for the ligands and arranged according to the final score function (S). (S), is the score of the last stage that was not set to none. The database browser was used for the visual examination of different poses the best ones were chosen. The 2D and 3D ligand interactions were obtained for each compound and different stereo views of the ligands inside the active site were saved as both MOE files and picture. Furthermore, Alignment of the synthesized compounds to folate inhibitor in the active site was also studied.
3 Results and discussion
3.1 Chemistry
Sulfonamides are considered as one of the main groups of drugs that target DHFR. The present exploration deals with the design and synthesis of some acetamide derivatives incorporating different thiazole-4-yl substituents (tails) to fulfil with the pharmacophore present in compounds that may exploit as DHFR inhibitors, conjugated with biologically active sulfonamide moiety to explore their combining effect on the antimicrobial and anticancer activities, and study their structure–activity relationship (Fig. 1). According to the significance point of view of 2-aminothiazole derivatives, numerous synthetic methodologies were formerly described. It's worthy to mention that, Hantzsch reaction of α-halocarbonyl compounds with thiourea affords a helpful method for the synthesis of 2-aminothiazoles (Hantzsch and Weber, 1887).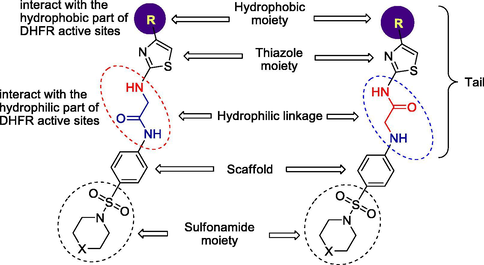
Structural components of the synthesized compounds in the DHFR enzymatic active site.
The preliminary key intermediates 2-amino-4-(substituted)-thiazoles 3a-f are attained via Hantzsch reaction of α-halocarbonyl derivatives 1a-f with thiourea in refluxing ethanol. The aminothiazole derivatives 3a-f were easily converted to the corresponding chloroacetamide derivatives 4a-f in excellent yield by reaction with chloroacetyl chloride in dimethylformamide (DMF) at room temperature (Scheme 1).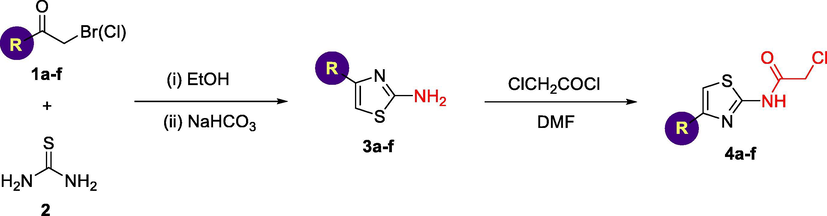
Synthesis of 2-chloro-N-(4-substituted-thiazol-2-yl)acetamides 4a-f.
€On the other hand, 4-(piperidin-1-ylsulfonyl)aniline (5a) and 4-(morpholin-4-ylsulfonyl)aniline (5b) were synthesized and transformed to the corresponding chloroacetamide derivatives 6a,b in excellent yields (up to 95%) according to our previous and reported procedure (Hussein, 2015; Hussein et al., 2019).
The intended compounds 2-(4-substituted-thiazol-2-ylamino)-N-(4-(piperidin-1-ylsulfonyl)-phenyl)acetamides 7a-f and N-(4-(morpholinosulfonyl)phenyl)-2-(4-substituted-thiazol-2-ylamino)-acetamides 7g-l, were acquired in good yields (51–94%) by reaction of chloroacetamide derivatives 6a and 6b, respectively, with 2-amino-4-(substituted)-thiazoles 3a-f in absolute ethanol for 5–8 h. (Scheme 2).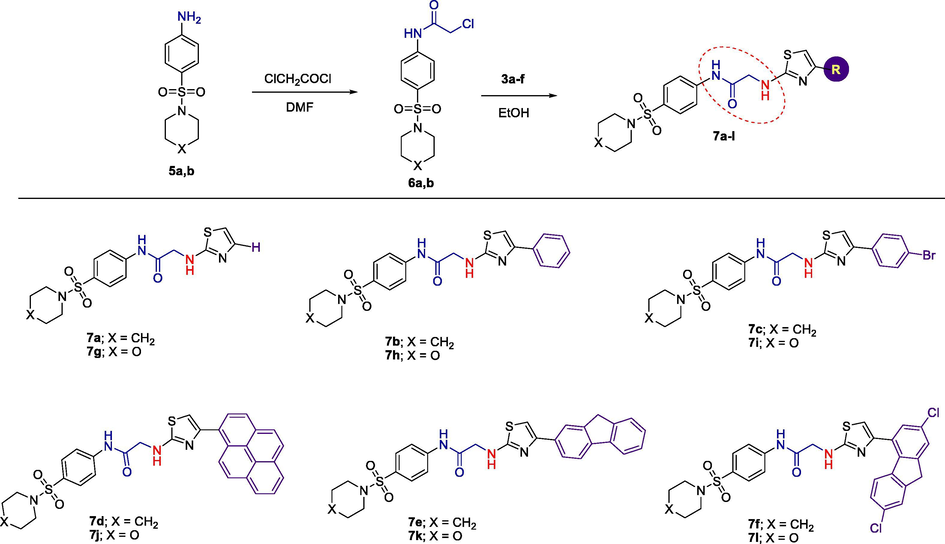
Synthesis of 2-(4-substituted-thiazol-2-ylamino)acetamides bearing sulfonamide moiety 7a-l.
On the other hand, reaction of 2-chloro-N-(4-substituted-thiazol-2-yl)acetamide 4a-f with sulfonamide derivatives 5a and 5b in ethanol under refluxing conditions afforded the target compounds N-(4-substituted-thiazol-2-yl)-2-(4-(piperidin-1-ylsulfonyl)phenylamino)acetamides 8a-f and 2-(4-(morpholinesulfonyl)phenylamino)-N-(4-substituted-thiazol-2-yl)acetamides 8g-l, respectively, in excellent yields (75–98%) (Scheme 3). The reactions were accomplished in the presence of triethylamine as a basic catalyst and reaction period of 5–9 h.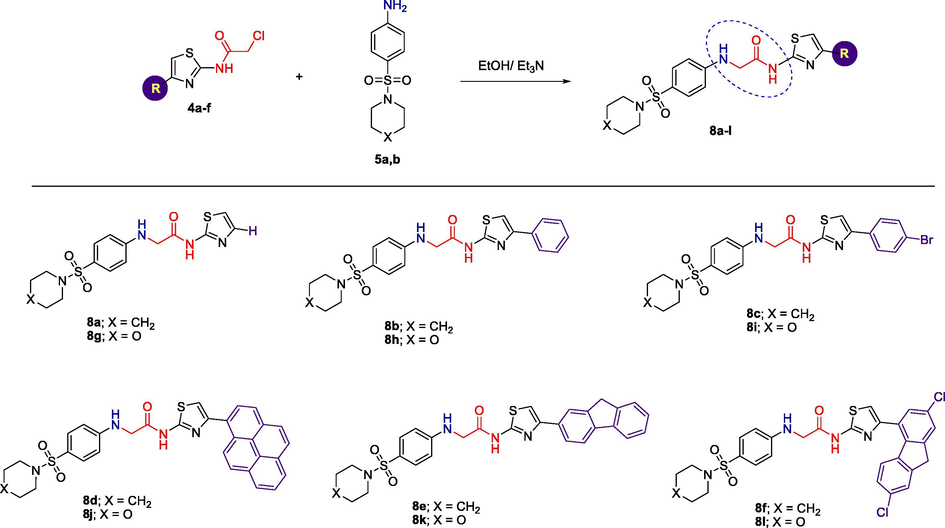
Synthesis of N-(4-substituted-thiazol-2-yl)acetamides bearing sulfonamide moiety 8a-l.
The chemical structures of newly synthesized compounds 7a-l and 8a-l well-confirmed on the basis of FT-IR, 1H NMR, 13C NMR, and DEPT-135 data (c.f. experimental section). The FT-IR spectra of compounds 7a-l showed the presence of characterized absorption bands at 3364–3284 and 3210–3110 cm−1 for two NH groups and 1701–1689 cm−1 for C⚌O groups. Furthermore, to full-establish the chemical structures of the synthesized compounds 1H, 13C, and DEPT-135 NMR were achieved in DMSO‑d6. As illustrative example, the 13C and DEPT-135 NMR spectra of 7c exhibited the presence of 16 signals (5 aromatics CH's, 6 aromatic quaternary carbons, 4 methylene carbons and one carbonyl carbon). Its 1H NMR spectrum revealed a downfield singlet signal at 10.79 ppm for NH proton, two doublets at 7.84 and 7.16 ppm (J = 8.0 Hz) for the protons of the scaffold moiety. In addition, multiplet at 7.75–7.74 and doublet signal at 7.54 ppm (J = 4.4 Hz) for the protons of 4-bromophenyl moiety and one NH proton, a singlet signal at 7.09 ppm for the thiazole ring proton. Additionally, a singlet signal at 4.33 ppm for the acetamido methylene protons and three multiples at 2.85–2.83, 1.53–1.51 and 1.35–1.33 ppm related piperidinyl ring protons.
However, the FT-IR spectra of 8a-l derivatives displayed characterized absorption bands at and 3365–3360 and 3215–3108 cm−1 for two NH groups and 1701–1643 cm−1 for C⚌O groups. The 1H NMR spectrum of 8i as a representative example, revealed two singlet signals at 12.44 and 6.12 ppm for two NH protons. Two doublets at 7.84 and 6.68 ppm (J = 8.0 Hz) for the protons of the scaffold moiety, in addition, two doublets at 7.62 and 7.34 ppm (J = 8.0 Hz) for 4-bromophenyl ring protons. A singlet signal at 7.74 ppm for the thiazole ring proton. Furthermore, a singlet signal at 4.43 ppm corresponding to the acetamido methylene protons and two triplet signals at 3.66, and 2.75 ppm (J = 4.4 Hz) for morpholinyl ring protons. In addition, its 13C and DEPT-135 NMR spectra revealed the presence of 15 signals for fifteen nonequivalent carbons (5 aromatics CH, 6 aromatic quaternary carbons, 3 methylene carbons and one carbonyl carbon.
3.2 Biological activity
3.2.1 Antimicrobial activity
The emergence of multidrug resistant microbial strains (MDRs) represents an alarming threat of public health concern worldwide (Qu et al., 2019). Consequently, the development of newly synthesized antimicrobial drugs is contemplated of global precedence especially with the prompt decline in the number of therapeutic opportunities. Although, sulfonamides were the first antimicrobial drug with selective toxicity against bacteria, they are not frequently used nowadays due to the rapid occurrence of resistance, the quite common side effects among treated patients as aplastic anemia and the availability of other efficient antimicrobials (Fair and Tor, 2014). The basis for the discerning toxic influence of sulfonamide on bacteria depends on the fact that mammalian cells are not reliant on endogenous synthesis of folic acid, instead, they have an independent unique folate uptake system that can use dietary folate via a transport system which is not present in most prokaryotes (Sköld, 2000). Consequently, in the present study 24 newly synthesized sulfonamide derivatives were evaluated for their antimicrobial activity against multidrug resistant strains of Gram-positive (S. aureus, MRSA and S. pneumoniae) and Gram-negative bacteria (E. coli, K. pneumoniae, P. aeruginosa and A. baumannii) as well as 3 fungal strains (A. flavus, A. niger and C. albicans) (c.f. supporting information).
Screening the antimicrobial activity for the newly synthesized sulfonamide derivatives was done by agar well diffusion assay (Ng and Freeze, 2015) using a concentration of 500 µg/mL. The results of the antimicrobial assay are given in Table 1. It is obviously observed from the obtained data that some of the newly synthesized sulfonamide derivatives showed comparatively high antimicrobial activity compared to the positive reference drugs, vancomycin for Gram-positive bacteria, gentamicin for Gram-negative bacteria, and fluconazole for fungi. The following compounds have given the best results in the inhibition of different types of multidrug resistant bacteria and fungi; compounds 7h, 7k and 8h showed relative high activity against S. aureus with a zone of inhibition (ZOI) value 18 mm, 17 mm and 16 mm, respectively. Compounds 7k and 8e achieved ZOI 16 mm and 14 mm, respectively against MRSA. Compound 8h exhibited moderate activity against S. pneumoniae with a ZOI value 9 mm. On the other hand, moderate activity was shown by compounds 7d, 7e and 7f against both Gram-negative and Gram-positive bacteria; against E. coli the ZOI value was 10 mm and 12 mm, respectively, however, higher activity was shown against S. aureus and MRSA with ZOI value 14 mm and 15 mm, respectively for compound 7f. Moreover, compounds 7b, 7h and 8h showed low activity against fungal strains with ZOI value 4 mm, 5 mm and 7 mm against A. flavus and 3 mm, 4 mm and 5 mm against C. albicans. A. niger showed low sensitivity for compound 8h with ZOI value 6 mm, therefore these derivatives have prospective for further inclusive studies.
Comp.
Gram (+ve) bacteriaa
Gram (−ve) bacteriaa
Fungia
S. aureus
MRSA
S. pneumoniae
E. coli
K. pneumoniae
P. aeruginosa
A. baumannii
A. flavus
A. niger
C. albicans
7a
8 ± 1
7 ± 0.3
–
–
–
–
–
–
–
–
7b
10 ± 0.5
–
–
–
–
–
7 ± 0.5
4 ± 0.7
–
3 ± 0.6
7c
10 ± 1
–
–
–
–
–
–
–
–
–
7d
14 ± 0.25
–
–
10 ± 0.4
–
–
–
–
–
–
7e
13 ± 0.3
13 ± 0.2
–
12 ± 0.7
–
–
–
–
–
–
7f
14 ± 1
15 ± 0.2
–
12 ± 0.1
–
–
–
–
–
–
7g
–
–
–
–
–
–
–
–
–
–
7h
18 ± 0.5
12 ± 0.6
–
–
–
–
5 ± 0.5
5 ± 0.5
–
4 ± 0.5
7i
9 ± 0.75
5 ± 0.3
–
–
–
–
–
–
–
–
7j
9 ± 0.6
5 ± 0.4
–
–
–
–
–
–
–
–
7k
17 ± 0.3
16 ± 0.9
–
–
–
5 ± 0.2
–
–
–
–
7l
8 ± 0.6
5 ± 0.8
–
–
–
–
3 ± 0.5
–
–
–
8a
–
–
–
–
–
–
–
–
–
–
8b
–
–
–
–
–
–
–
–
–
–
8c
8 ± 0.5
13 ± 0.6
–
–
–
–
–
–
–
–
8d
10 ± 0.3
8 ± 0.7
–
–
–
–
–
–
–
–
8e
12 ± 0.4
14 ± 0.6
–
–
–
–
–
–
–
–
8f
11 ± 0.6
9 ± 0.9
–
–
–
–
5 ± 0.2
–
–
–
8g
–
–
–
–
–
–
–
–
–
–
8h
16 ± 0.4
12 ± 0.3
9 ± 0.7
–
–
–
–
7 ± 0.6
6 ± 0.5
5 ± 0.4
8i
11 ± 0.3
7 ± 0.2
–
–
–
–
–
–
–
–
8j
9 ± 0.8
9 ± 0.6
–
–
–
–
–
–
–
–
8k
14 ± 0.9
14 ± 0.5
–
–
–
–
–
–
–
–
8l
14 ± 0.5
10 ± 0.3
–
–
–
–
–
–
–
–
Pcb
28 ± 1
26 ± 0.6
20 ± 0.4
28 ± 1
20 ± 0.4
30 ± 1
20 ± 0.5
18 ± 0.5
18 ± 0.5
20 ± 0.4
Additionally, it is noticeably evident from the antimicrobial results that the newly synthesized sulfonamide derivatives 7h and 8h demonstrate dual activities and can be considered as promising antibacterial and antifungal agents. Also, from all the collected data, it could be concluded that, the following compounds 7d, 7e, 7f, 7h, 7k, 8e and 8h are the most active newly synthesized sulfonamide derivative exclusively against Gram-positive bacteria.
The minimum inhibitory concentration (MIC) of the most active newly synthesized sulfonamide derivatives was determined and reported in Table 2. The MIC varied within the range (500 µg/mL–7.8 µg/mL). Compound 7d, 7e, 7f, 7h, 7k, 8e and 8h were potent against Gram-positive bacteria (S. aureus, MRSA and S. pneumoniae) with an MIC ranged from (31.25–62.5 µg/mL). On the other hand compound 7d, 7f achieved moderate activity against Gram-negative bacteria (E. coli) with an MIC ranged from (62.5–125 µg/mL). All results were compared to vancomycin and gentamycin as antibacterial reference drug. Noteworthy, a relatively high MIC values, and this may be attributed to the nature of the tested microbial strains as they were multidrug resistant particularly for trimethoprim/sulfamethoxazole 25 µg. Pc: positive control is Vancomycin (50–0.78 µg/mL) for Gram-positive bacteria and Gentamycin (10–0.15 µg/mL) for Gram-negative bacteria, DMSO was the negative control. (–): MIC value was >500 µg/mL. All experiments were repeated three times and average means were represented.
Comp.
Test strain/MIC (µg/mL)
S. aureus
MRSA
S. pneumoniae
E. coli
7d
62.5
–
–
125
7e
62.5
62.5
–
125
7f
31.25
62.5
–
62.5
7h
31.25
62.5
–
–
7k
31.25
31.25
–
–
8e
62.5
31.25
–
–
8h
31.25
62.5
125
–
Pc
0.7
0.7
0.7
0.3
DMSO
–
–
–
–
Compounds 7f, 7h, 7k, 8e and 8h were the most potent sulfonamide derivatives against the multidrug resistant tested microbial strains in this study. In conclusion, with the worsening situation of the widespread antibiotic resistance globally, the newly synthesized sulfonamide derivatives could re-attract the interest in treatment of serious infectious disease caused by multidrug resistant microbial strains.
4 In vitro anticancer activity
4.1 Cell viability screening
All new tested compounds exert a prominently cytotoxic influence on the WI-38 normal lung fibroblast cells, A549 lung cancer cells, and MDA-MB-231 breast cancer cells. Taxol a well-known chemotherapeutic agent (IC50 = 41, 2.30, and 40 µg/mL for WI-38, A549, and MDA-MB-231, respectively) was used as the reference control. The cell viability of WI-38 under different concentration (c.f. Supporting information files) and the IC50 (the concentration which inhibit the 50% of cell populations) calculations for each compound, clearly show that all tested compounds induce a significant cell death except 8a having N-(thiazol-2-yl)acetamide, 8d possessing N-(4-(pyren-1-yl)thiazol-2-yl)acetamide, 8e having (4-(9H-fluoren-2-yl)thiazol-2-yl)acetamide, 8f bearing (4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)acetamide of piperidinosulfonyl moiety, 8g bearing N-(thiazol-2-yl)acetamide, and 8k having (4-(9H-fluoren-2-yl)thiazol-2-yl)acetamide of morpholinosulfonyl moiety with IC50 value of 1610, 1966, 2959, 1734, 933, and 1001 µg/mL, respectively. Moving to A549 and MDA-MB-231 cancer cells, cell viability and IC50 calculations prove that the 24 sulfonamide derivatives have an obvious cell death except compound 8g with IC50 value of 1137 and 1378 µg/mL, respectively. The obtained IC50 of the tested compounds against WI-38, A549, and MDA-MB-231 cell lines are presented in Table 3. From screening results shown in Table 3, it can figure out that compounds 8d (IC50 = 36.10 and 12.48, respectively), 8e (IC50 = 16.10 and 72, respectively), and 8f (IC50 = 22.10 and 72, respectively) have a potential anti-cancer activity compared with Taxol (Fig. 2). IC50 value: concentration causing 50% inhibition of cell viability. Mean of three results obtained from three experiments ± SD.
Comp.
R
X
IC50 (µg/mL)
WI-38
A549
MDA-MB-231
7a
H
CH2
87 ± 0.8
29.1 ± 0.2
48 ± 0.4
7b
C6H5
CH2
162 ± 0.8
134 ± 0.8
50.4 ± 0.1
7c
4-BrC6H4
CH2
95 ± 0.9
21 ± 0.6
107.8 ± 0.4
7d
Pyren-1-yl
CH2
106 ± 0.8
194 ± 0.6
78.7 ± 0.8
7e
9H-fluoren-2-yl
CH2
545 ± 0.9
130.5 ± 0.4
176.7 ± 0.5
7f
2,7-Dichloro-9H-fluoren-4-yl
CH2
382 ± 0.9
36.1 ± 0.3
54.2 ± 0.8
7g
H
O
572 ± 1.2
140.5 ± 0.2
194 ± 0.9
7h
C6H5
O
229 ± 0.9
145.6 ± 0.4
182 ± 0.2
7i
4-BrC6H4
O
166 ± 0.4
294 ± 1.1
50 ± 0.4
7j
Pyren-1-yl
O
109 ± 0.9
188.9 ± 0.4
91 ± 0.5
7k
9H-fluoren-2-yl
O
596 ± 0.8
149 ± 0.5
366.4 ± 0.4
7l
2,7-Dichloro-9H-fluoren-4-yl
O
407 ± 0.9
55.2 ± 0.3
105 ± 0.4
8a
H
CH2
1610 ± 0.9
121 ± 0.4
685 ± 0.2
8b
C6H5
CH2
257 ± 0.4
18.1 ± 0.2
43.4 ± 0.1
8c
4-BrC6H4
CH2
207 ± 0.4
1020 ± 0.8
39.44 ± 0.4
8d
Pyren-1-yl
CH2
1966±0.4
36.1±0.2
12.48±0.2
8e
9H-fluoren-2-yl
CH2
2959±0.8
16.1±0.3
72±0.6
8f
2,7-Dichloro-9H-fluoren-4-yl
CH2
1734±0.4
22.1±0.4
72±0.8
8g
H
O
933.4 ± 1.2
1137 ± 0.4
1378 ± 0.4
8h
C6H5
O
79 ± 0.9
335 ± 0.5
194 ± 0.4
8i
4-BrC6H4
O
103 ± 0.8
171 ± 0.6
43.7 ± 0.3
8j
Pyren-1-yl
O
121 ± 0.4
169 ± 0.4
258 ± 0.5
8k
9H-fluoren-2-yl
O
1001 ± 0.9
558 ± 0.8
75 ± 0.4
8l
2,7-Dichloro-9H-fluoren-4-yl
O
729 ± 0.8
147 ± 0.6
647 ± 0.4
Taxol
41 ± 0.4
2.30 ± 0.4
40 ± 0.4
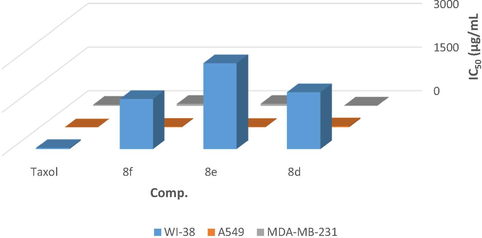
IC50 of the most active compounds 8d, 8e and 8f against WI-38, A549, and MDA-MB-23 in comparison with Taxol.
4.1.1 Fluorescence-activated cell sorting (FACS) analysis
In addition to the preceding reported data which elucidate the effect of sulfonamide derivatives on the biology of the cell (Payne et al., 2008; Fukuoka et al., 2001; Villar et al., 2004; Supuran et al., 2001; Casini et al., 2002; Huang et al., 2006; Kawai et al., 2006; Kenneth et al., 2006), further studies are developed to confirm the cytotoxicity results for the most promised compounds 8d, 8e, and 8f, and to expose the mechanism of cell death by using FACS analysis. For the normal cell line, the results clearly showed that, compounds 8d and 8f induced a moderate necrotic cell death (15.3% and 27.8% of total cell number); accordingly, while 8e derivative induced a necrotic cell death only for 1.65% of the total cell number. Both compounds 8d and 8f derivatives have not an observed apoptotic cell death with (1.37% and 0.89% of total cell number) while 8e has a moderate apoptotic cell death with (19.17% of the total cell number) as shown in Fig. 3.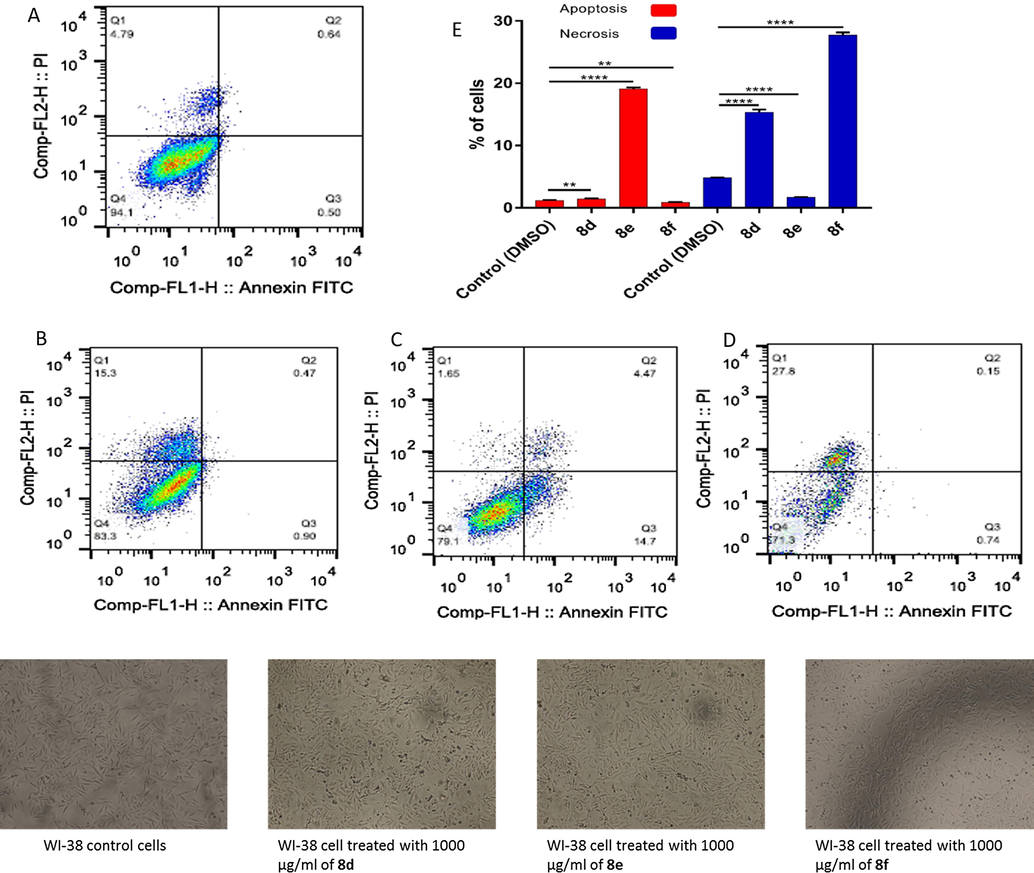
Apoptotic and necrotic cell death were assessed using Annexin V and Probidium Iodide (PI) staining and analyzed using flow cytometer after 24 h treatment of sulfonamide derivatives. (A) WI-38 cells control (DMSO), (B) WI-38 cell treated with 1000 µg/mL of 8d, (C) WI-38 cell treated with 1000 µg/mL of 8e, (D) WI-38 cell treated with 1000 µg/mL of 8f, and (E) quantification of apoptotic and necrotic cell death for each drug on WI-38 cells, * indicate significant difference (P value < 0.05), **** indicate significant difference (P value < 0.0001).
In case of A549 and MDA-MB-31 cancer cell lines, there is not observed necrotic cell death when the cells treated with the IC50 from each compound except 8e and 8f which induced 8.67% and 8.51%, respectively, in MDA-MB-31 cells (Figs. 4 and 5). On the other hand, the three sulfonamide derivatives 8d, 8e, and 8f revealed a high significant apoptotic cell death with 44.62%, 58.18%, and 18.50% of the total A549 cell populations as shown in Fig. 4, while they induced 47.45, 42.40%, and 56.30% of the total MDA-MB-231 cell populations as shown in Fig. 5.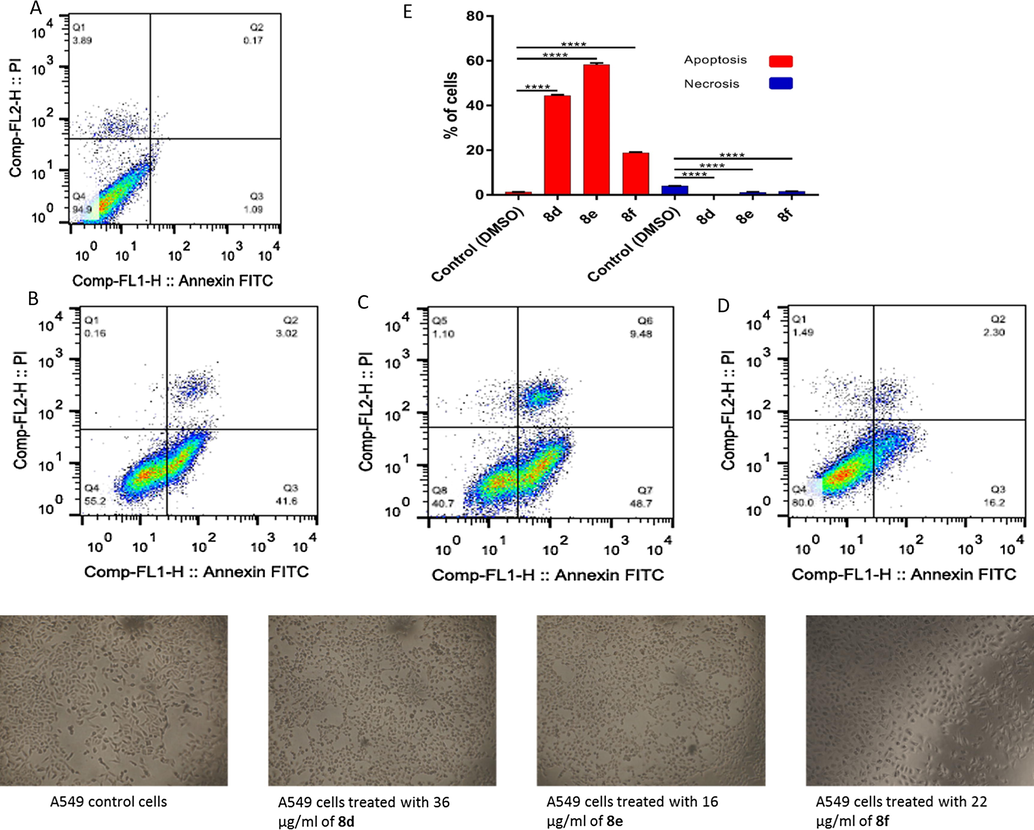
Apoptotic and necrotic cell death were assessed using Annexin V and Probidium Iodide (PI) staining and analyzed using flow cytometer after 24 h treatment with sulfonamide derivatives. (A) A549 cells control (DMSO), (B) A549 cell treated with 36 µg/mL of 8d, (C) A549 cell treated with 16 µg/mL of 8e, (D) A549 cell treated with 22 µg/mL of 8f, and (E) quantification of apoptotic and necrotic cell death for each drug on A549 cells, * indicate significant difference (P value < 0.05), **** indicate significant difference (P value < 0.0001).
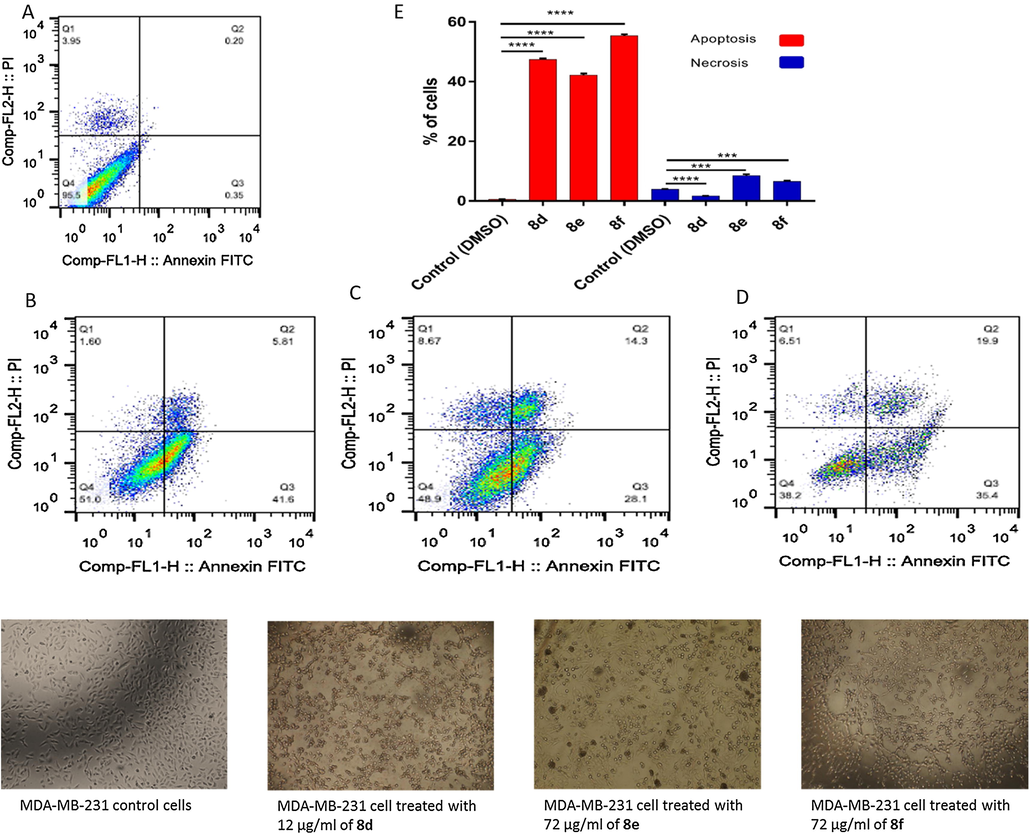
Apoptotic and necrotic cell death were assessed using Annexin V and Probidium Iodide (PI) staining and analyzed using flow cytometer after 24 h treatment with sulfonamide derivatives. (A) MDA-MB-231 cells control (DMSO), (B) MDA-MB-231 cell treated with 12 µg/mL of 8d, (C) MDA-MB-231 cell treated with 72 µg/mL of 8e, (D) MDA-MB-231 cell treated with 72 µg/mL of 8f, and (E) quantification of apoptotic and necrotic cell death for each drug on MDA-MB-231 cells, * indicate significant difference (P value < 0.05), **** indicate significant difference (P value < 0.0001).
4.1.2 Docking and Molecular modeling
In the current work, docking study was done in order to understand the mechanism of interaction of the synthesized compounds 7a-l and 8a-l with DHFR to authenticate the difference in activity as antibacterial and anticancer between different synthesized analogues. After preparation of the enzyme, redocking of the co-crystallized folate ligand was done (Fig. 6) with different placement protocol in order to the best methodology for docking. The alpha triangle placement method showed RMSD value of<2 (0.3581) which indicate the confidence in the produced docking results. As can be seen from the 2D and 3D interaction between folate and DHFR enzyme, there are some important amino acid residues that share in the binding between folate and DHFR active site. There are five hydrogen bonds: two with Glu30, two with Arg70 and one with Asn64 beside hydrophobic interaction with Phe31, Phe34 and Ile60.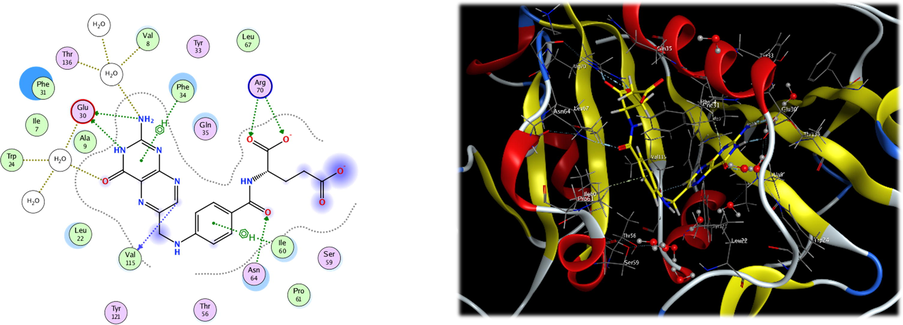
2D and 3D interaction of folate and DHFR enzyme.
Molecular docking of the conformation database of all synthesized compounds into the active site of DHFR was carried out using the mentioned protocol with the results refinement using forcefield based scoring function GBVI/WSA dG which estimates the free energy of binding of the ligand from a given pose.
The functional form is a sum of terms:
c
Represents the average gain/loss of rotational and translational entropy.
α, β
Are constants which were determined during training (along with c), and forcefield dependent, respectively.
Ecoul
The coulombic electrostatic term which is calculated using currently loaded charges, using a constant dielectric of 1.
Esol
The solvation electrostatic term which is calculated using the GB/VI solvation model.
Evdw
The van der Waals contribution to binding.
SAweighted
The surface area, weighted by exposure.
The details of the interactions are as the following: Compound 7f interacts with the active site through formation of hydrogen bond between the carbonyl of the carboxamide group and Asn64 with a distance of 2.99 Ǻ, π-H bond between the thiazole ring and Ile60 with a distance of 4.9 Ǻ and π- π bond between fluorene ring and Phe34 with a distance of 3.96 Ǻ. Compound 7h interacts with the active site through formation of hydrogen bond between the sulfonamide group and Asn64 with a distance of 2.85 Ǻ beside hydrophobic interactions with different amino acid residues like Phe31, Phe34, Ile0 and Arg70. Compound 7k interacts with the active site through formation of two hydrogen bonds between thiazole ring and Gln35 and Asn64 with distances of 4.25 and 3.35 Ǻ, respectively, beside π-H bond between the benzene ring and Ile60 with a distance of 3.81 Ǻ. Compound 8e interacts with the active site through formation of two π-H bonds between benzene ring next to the sulfonyl group and Phe34 and Ile60 with distances of 4.24 and 4.13 Ǻ, respectively. Compound 8h interacts with the active site through formation of hydrogen bond between thiazole ring and Val115 with a distance of 3.23 Ǻ and π- π bond between the benzene ring adjacent to the sulfonyl group and Phe31 with a distance of 3.88 Ǻ. In general, beside the interactions mentioned above, most the compounds showed hydrophobic interaction with different amino acid residue in the vicinity of the active site (Fig. 7). Upon examining the scoring results, we can concluded that, it was found that most of the highest active compounds showed better scores. So, compounds 7f, 7h, 7k, 8e and 8h showed high scores in comparison with other analogues. The scores were in the range of −8.5810–7.8628 Kcal/mole.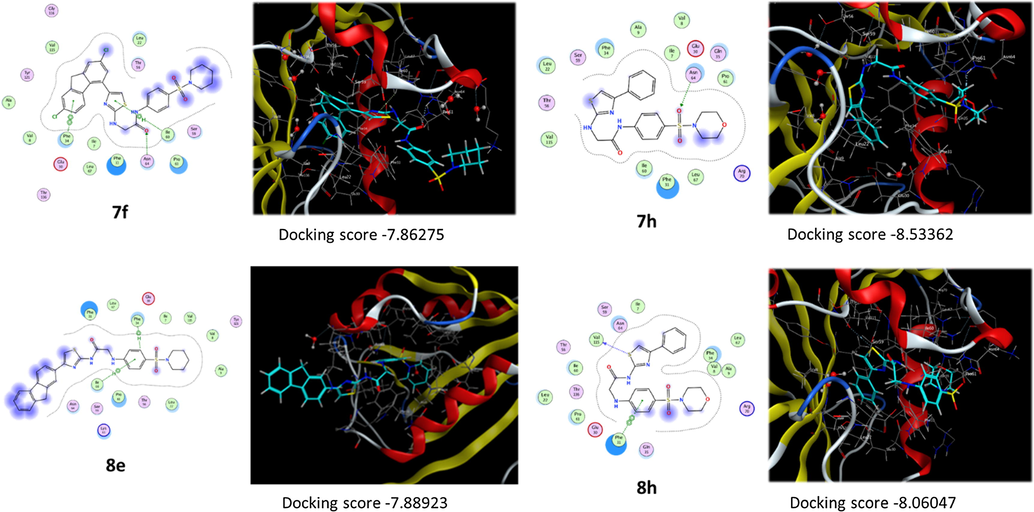
2D and 3D representation of compounds 7f, 7h, 8e, and 8h interaction with DHFR enzyme.
Furthermore, in order to interpret the activity of the most active compounds, overlay of the most active compounds to folate in the vicinity of active site, as can be seen in an example of compound 7h, showed good alignment of the sulfonamide analogues and folate where the sulfamoyl moiety and the anilino amino group of the synthesized compounds overlays the amide linkage between the glutamyl and benzoyl moieties and the anilino amino group of folate molecules respectively (Fig. 8).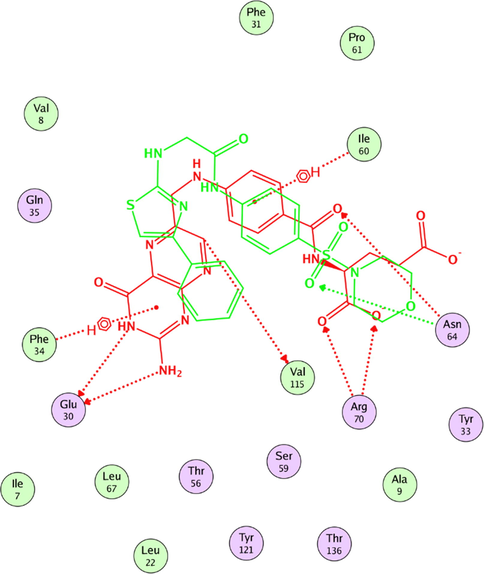
Overlay of compound 7h to folate in the vicinity of active site.
5 Conclusion
Two new series of N4-substituted sulfonamide derivatives bearing thiazole moiety 7a-l and 8a-l were synthesized as hybrid structures and evaluated for their antimicrobial activity against multidrug resistant strains (MDRs) and cytotoxic activity against normal lung fibroblast (WI-38), human lung carcinoma (A549), and human breast carcinoma (MDA-MB-231) cell lines. Some of these compounds showed remarkable antimicrobial activity against MDRs strains, compounds 7h and 8h exhibited dual activity as potent antibacterial and antifungal agents. Compounds 8d, 8e, and 8f showed selective cytotoxic activity against A549 and MDA-MB-231 cancer cell lines. The mechanism of antimicrobial and anticancer activities have investigated using FACS analysis and molecular docking simulation using MOE software. To the best of our knowledge, these obtained multi-addressable biological potentials of these new synthesized sulfonamides reported in this work may open a new era in the field of medicinal chemistry.
Acknowledgment
The Authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University, Saudi Arabia for the financial support through the project number 18-SCI-1-01-0009.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A green synthetic approach to the synthesis of Schiff bases from 4-amino-2-thioxo-1,3-diazaspiro[5.5]undec-4-ene-5-carbonitrile as potential anti-inflammatory agents. Russ. J. Bioorg. Chem.. 2014;40:343-349.
- [Google Scholar]
- Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur. J. Med. Chem.. 2010;45:1849-1853.
- [Google Scholar]
- Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur. J. Med. Chem.. 2010;45:738-744.
- [Google Scholar]
- Synthesis and in vitro anticancer screening of some novel 4-[2-amino-3-cyano-4-substituted-5,6,7,8-tetrahydro-quinolin-1-(4H)-yl]benzenesulfonamides. Eur. J. Med. Chem.. 2010;45:3011-3018.
- [Google Scholar]
- Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur. J. Med. Chem.. 2011;46:201-207.
- [Google Scholar]
- Ammonium chloride catalyzed synthesis of novel Schiff bases from spiro[indoline-3,4′-pyran]-3′-carbonitriles and evaluation of their antimicrobial and anti-breast cancer activities. SpringerPlus. 2016;5:887.
- [Google Scholar]
- Opposite effects of hypoglycemic and hyperglycemic sulfonamides upon ionophore-mediated calcium transport. Biochem. Pharmacol.. 1980;29:1879-1882.
- [Google Scholar]
- Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. Arkivoc. 2009;6:89-102.
- [Google Scholar]
- Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules. 2010;15:2028-2038.
- [Google Scholar]
- Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem.. 1995;38:4929-4936.
- [Google Scholar]
- Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides. Eur. J. Med. Chem.. 2011;46:6066-6074.
- [Google Scholar]
- Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem.. 2010;45:651-660.
- [Google Scholar]
- Dihydrofolate reductase. In: Blakley R.L., Benkovic S.J., eds. Folates and Pteridines. New York: Wiley; 1984. p. :191-253.
- [Google Scholar]
- Exploring the molecular mechanism of dihydrofolate reductase. Faraday Discuss.. 1992;93:217-224.
- [Google Scholar]
- Sulfonamide derivatives with protease inhibitory action as anticancer, anti-inflammatory and antiviral agents. Exp. Opin. Ther. Pat.. 2002;12:1307-1327.
- [Google Scholar]
- CLSI, 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved Standard, 9th ed., CLSI document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem.. 2018;144:874-886.
- [Google Scholar]
- Antitumor and immunomodulatory activities of thiosemicarbazones and 1,3-thiazoles in Jurkat and HT-29 cells. Biomed. Pharmacother.. 2016;82:555-560.
- [Google Scholar]
- Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem.. 2014;6:25-64.
- [Google Scholar]
- Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Invest. New Drugs. 2001;19:219-227.
- [Google Scholar]
- Evaluation of the local anaesthetic activity of 3-aminobenzo[d]isothiazole derivatives using the rat sciatic nerve model. Eur. J. Med. Chem.. 2009;44:473-481.
- [Google Scholar]
- Novel 4-(4-substituted-thiazol-2-ylamino)-N-(pyridin-2-yl)-benzenesulfonamides as cytotoxic and radiosensitizing agents. Arch. Pharm. Res.. 2012;35:59-68.
- [Google Scholar]
- Anticancer and radio-sensitizing evaluation of some new thiazolopyrane and thiazolopyranopyrimidine derivatives bearing a sulfonamide moiety. Eur. J. Med. Chem.. 2011;46:5120-5126.
- [Google Scholar]
- The large scale antibacterial, antifungal and anti-phage efficiency of Petamcin-A: new multicomponent preparation for skin diseases treatment. Ann. Clin. Microbiol. Antimicrob.. 2015;14:28.
- [Google Scholar]
- Synthesis of N-(6-arylbenzo[d]thiazole-2-acetamide derivatives and their biological activities: an experimental and computational approach. Molecules. 2016;21:266-282.
- [Google Scholar]
- Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe) Ber. Dtsch. Chem. Ges.. 1887;20:3118-3132.
- [Google Scholar]
- Dihydrofolate reductase inhibitors as antibacterial agents. Biochem. Pharmacol.. 2006;71:941-948.
- [Google Scholar]
- Synthesis and evaluation of N-acyl sulfonamides as potential prodrugs of cyclin-dependent kinase inhibitor JNJ-7706621. Bioorg. Med. Chem. Lett.. 2006;16:3639-3641.
- [Google Scholar]
- Ammonium chloride-catalyzed four-component sonochemical synthesis of novel hexahydroquinolines bearing a sulfonamide moiety. Russ. J. Org. Chem.. 2015;51:54-64.
- [Google Scholar]
- Regioselective synthesis and anti-inflammatory activity of novel dispiro[pyrazolidine-4,3'-pyrrolidine-2',3“-indoline]-2”,3,5-triones. Arkivoc. 2011;10:85-98.
- [Google Scholar]
- Design, synthesis, and biological evaluation of novel N4-substituted sulfonamides: acetamides derivatives as dihydrofolate reductase (DHFR) inhibitors. BMC Chem.. 2019;13:91.
- [Google Scholar]
- Synthesis of some novel 6′-(4-chlorophenyl)-3,4′-bipyridine-3′-carbonitriles: assessment of their antimicrobial and cytotoxic activity. Z. Naturforsch.. 2015;70b:783-795.
- [Google Scholar]
- Efficient synthesis and antimicrobial evaluation of some Mannich bases from 2-arylidine-1-thia-4-azaspiro[4.5]decan-3-ones. Chem. Cent. J.. 2015;9:25.
- [Google Scholar]
- Topological modeling of lipophilicity, diuretic activity, and carbonic inhibition activity of benzene sulfonamides: a molecular connectivity approach. Bioorg. Med. Chem. Lett.. 2004;14:5661-5666.
- [Google Scholar]
- Development of sulfonamide compounds as potent methionine aminopeptidase type II inhibitors with antiproliferative properties. Bioorg. Med. Chem. Lett.. 2006;16:3574-3577.
- [Google Scholar]
- The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: results of a phase 1 study. Clin. Cancer Res.. 2006;12:2834-2840.
- [Google Scholar]
- Resazurin Microtiter Assay Plate method for detection of susceptibility of multidrug resistant Mycobacterium tuberculosis to second-line anti-tuberculous drugs. Egyp. J. Chest Dis. Tubercul.. 2013;62:241-247.
- [Google Scholar]
- Synthesis and evaluation of sulfonamide-bearing thiazole as carbonic anhydrase isoforms hCA I and hCA II. J. Enzyme Inhib. Med. Chem.. 2016;31:1300-1305.
- [Google Scholar]
- Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur. J. Med. Chem.. 2012;50:433-440.
- [Google Scholar]
- Synthesis of polyhydroxylated aromatic mandelic acid amides and their antioxidative potential. Tetrahedron. 2001;57:1277-1282.
- [Google Scholar]
- Discovery of novel 2-N-aryl-substituted benzenesulfonamidoacetamides: orally bioavailable tubulin polymerization inhibitors with marked antitumor activities. Chem. Med. Chem.. 2012;7:680-693.
- [Google Scholar]
- Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur. J. Med. Chem.. 2009;44:678-688.
- [Google Scholar]
- Molecular modeling, synthesis, antibacterial and cytotoxicity evaluation of sulfonamide derivatives of benzimidazole, indazole, benzothiazole and thiazole. Bioorg. Med. Chem.. 2018;26:3414-3428.
- [Google Scholar]
- Human genetic disorders involving glycosylphosphatidylinositol (GPI) anchors and glycosphingolipids (GSL) J. Inherit. Metab. Dis.. 2015;38:171-178.
- [Google Scholar]
- In Protease inhibitors in AIDS therapy. Basel, New York: Marcel Dekker; 2001. p. :200-250.
- Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides, derivatives of 4-phenyl-piperazine. Farmaco. 2005;60:969-973.
- [Google Scholar]
- Identification of KD5170: a novel mercaptoketone-based histone deacetylase inhibitor. Bioorg. Med. Chem. Lett.. 2008;18:6093-6096.
- [Google Scholar]
- Crisis of Antimicrobial Resistance in China: Now and the Future. Front. Microbiol.. 2019;10:2240.
- [Google Scholar]
- Synthesis, pharmacological evaluation and docking studies of N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl)acetamide analogs as COX-2 inhibitors. Bioorg. Med. Chem. Lett.. 2012;22:820-823.
- [Google Scholar]
- Anti-liver cancer activity in vitro and in vivo induced by 2-pyridyl 2,3-thiazole derivatives. Toxicol. Appl. Pharmacol.. 2017;329:212-223.
- [Google Scholar]
- Sulfonamide resistance: mechanisms and trends. Drug Resist. Updat.. 2000;3:155-160.
- [Google Scholar]
- Inhibition of herpes simplex virus replication by a 2-aminothiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J. Virol.. 1998;72:6979-6987.
- [Google Scholar]
- Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg. Med. Chem.. 2001;9:703-714.
- [Google Scholar]
- Carbonic anhydrase inhibitors and their therapeutic potential. Expert. Opin. Ther. Pat.. 2000;10:575-600.
- [Google Scholar]
- Isosterism and molecular modification in drug design. Chem. Soc. Rev.. 1979;8:563-580.
- [Google Scholar]
- Synthesis and cytotoxic activity of lipophilic sulphonamide derivatives of the benzo[b]thiophene 1,1-dioxide. Bioorg. Med. Chem.. 2004;12:963-968.
- [Google Scholar]
- Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc.. 2008;3:163-175.
- [Google Scholar]
- Rational design, synthesis, and biological activity of N-(1,4-benzoxazinone)acetamide derivatives as potent platelet aggregation inhibitors. Bull. Korean Chem. Soc.. 2018;39:146-155.
- [Google Scholar]
- Dimethyl-diphenyl-propanamide derivatives as nonsteroidal dissociated glucocorticoid receptor agonists. J. Med. Chem.. 2010;53:8241-8251.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.03.014.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2
Supplementary data 3
Supplementary data 3
Supplementary data 4
Supplementary data 4







