Translate this page into:
Tb2CoMnO6 double perovskites nanoparticles as photocatalyst for the degradation of organic dyes: Synthesis and characterization
⁎Corresponding author. Salavati@kashanu.ac.ir (Masoud Salavati-Niasari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The importance of water is so high that new methods and materials are constantly being developed to increase freshwater quality. For this purpose, to investigate the unique properties of double perovskites in the photocatalytic process, novel Tb2CoMnO6 nanoparticles were synthesized using the sol-gel auto-combustion method. In this method, maltose, cellulose, and Date syrup were used as fuel. Two different ratios of maltose were tested to investigate the effect of fuel content on size and morphology. The synthesized particles in the presence of maltose in an equal ratio to the precursor were selected as the optimal sample by using different analyses such as X-ray diffraction (XRD) and scanning electron microscopy (SEM). Other properties of optimal nanoparticles were investigated using transmission electron microscopy (TEM), energy dispersive spectrometry (EDS), Fourier transform infrared (FTIR), vibrating sample magnetometer (VSM) and diffuse reflectance analysis (DRS) analysis. These nanoparticles were used to investigate the photocatalytic properties under UV light in the aqueous solution of Eriochrome black T, Rhodamine B, Thymol blue, and Acid red dyes. The results of the photocatalytic process showed 70% discoloration for the Eriochrome Black T. Three compounds, include Ethylenediaminetetraacetic acid, 1,4-Benzoquinone, and Benzoic acid, were used as scavengers to investigate the mechanism of the photocatalytic process. The results showed that the mechanism of the photocatalytic process proceeds through superoxide radicals, which Benzoquinone scavenges.
Keywords
Tb2CoMnO6
Double perovskite
Nanoparticles
Sol-gel
Auto-combustion
Photocatalyst
1 Introduction
Freshwater has long been one of the main problems for human life, and efforts to access water resources have always been ongoing (Iglesias, 2020). Today, humans are facing a decrease in freshwater resources (Natalia et al., 2020). Therefore, finding new and cost-effective solutions for managing, purifying, and reusing water is at the forefront of scientific projects, and progress in these areas is constantly occurring (Gomes et al., 2019; Mukhopadhyay et al., 2020; Chaplin, 2019). The photocatalysis process is one of the most important and valuable methods for water treatment.
The photocatalysis is a process that removes contaminants in water under visible or UV light in the presence of a light-sensitive compound (Al-Mamun et al., 2019). Semiconductors are light-sensitive materials that cause electron-hole pairs in the environment when light hits them due to electron transfer (Zhu and Zhou, 2019). The electron-hole created in the aqueous medium leads to the production of free radicals. The interaction of these free radicals with water pollution causes their removal (Koe et al., 2020). Semiconductor materials include a wide range of compounds. One of these large families is the perovskites (Mitzi, 2019).
The structure of perovskite includes a family of materials with different properties and applications (Assirey, 2019). Oxide-type inorganic perovskites are materials of interest for use in many applications, including solar cells, sensors, catalysts, and as anodes in lithium-ion batteries (Jung and Park, 2015; George et al., 2020; Garcia-Muñoz et al., 2019; Chang et al., 2021). From this large family, double perovskites with the structure M2ABO6 (M is an element of lanthanides, A, and B of transition metals) have recently attracted the attention of many researchers due to their unique ferromagnetic and optical properties with direct band-gap. Various applications have been reported for these materials, such as La2NiMnO6 with application in solar-cell, Gd2CoMnO6 with dielectric properties, and La2MnTiO6 used as photocatalyst (Sariful Sheikh et al., 2017; Silva et al., 2019; Shirazi et al., 2020). These various properties and applications promise the potential of these materials in different processes.
Various methods, include ultrasonic, sol–gel, and co-precipitation, have been reported in the literature to synthesize double perovskites (Baladi et al., 2019; Dara et al., 2021; Wu et al., 2013). For example, Sivasamy et al. Synthesized Gd2MnFeO6 nanoparticles by the sol–gel method, and the optical and magnetic properties of these nanoparticles were investigated (Sivasamy et al., 2020). In this work, the sol–gel auto-combustion method was used for the synthesis of nanoparticles. Tb2CoMnO6 double perovskite nanoparticles were synthesized for the first time. Different fuels were used to reach the appropriate size of nanoparticles. For the first time, Date syrup was used as natural fuel for the synthesis of nanoparticles. The ultimate goal was to evaluate the efficiency of these nanoparticles in the photocatalysis process as a potential candidate. The photocatalytic activity of these nanoparticles and the mechanism of the photocatalysis process was investigated for the first time.
2 Experimental
2.1 Material and characterization
All the chemicals used in this work were commercially available and employed without further purification. Tb(NO3)3·6H2O with a molecular weight of 453.03 g.mol−1, Mn(NO3)2·4H2O with a molecular weight of 251.01 g.mol−1, Co(NO3)2·6H2O with a molecular weight of 291.03 g.mol−1 with a purity of over 99% were purchased from Merck company. A diffractometer of the Philips company with X’PertPro monochromatized Cu Kα radiation (λ = 1.54 Å) was used to collect XRD (X-ray diffraction) patterns to confirm the type of structure and check the purity of synthesized nanoparticles. The microscopic morphology of the products was studied by FESEM (field emission scanning electron microscopy) (Mira3 tescan). EDS (energy dispersive spectrometry) analysis was studied by XL30, Philips microscope. Transmission electron microscopy (TEM) image was achieved via a Philips EM208 transmission electron microscope with an accelerating voltage of 200 kV. Fourier transform infrared (FTIR) spectra were detected by means of Nicolet Magna-550 spectrometer in KBr pellets The UV–vis diffuse reflectance analysis was done by applying a UV–vis spectrophotometer (Shimadzu, UV2550, Japan). Magnetic properties were measured using VSM (vibrating sample magnetometer) (Meghnatis. Daghigh Kavir Co.; Iran).
2.2 Synthesis procedure
A sol–gel auto-combustion method with a ratio of 2: 1 Tb(NO3)3·6H2O (2 mmol) to Co(NO3)2·6H2O (1 mmol) and Mn(NO3)2·6H2O (1 mmol) in the presence of maltose, cellulose, and date syrup was used as fuel for the synthesis of Tb2CoMnO6 double perovskite nanoparticles. All salts were dissolved separately in distilled water. Fuel dissolved in distilled water was added to the solution containing terbium on the stirrer. The other two solutions containing cobalt and manganese were added to terbium and fuel after mixing. The resulting solution was completely homogenized on a stirrer for 50 min at 50 °C. Then the temperature increased to 110 °C, and after 30 min and combustion, a soft powder was obtained. The resulting powder was calcined at 1000, 900 and 800 °C. Different conditions were adopted for synthesis, as shown in Table 1.
No.
Mole ratio of Tb:Co:Mn
Fuel
Ratio of fuel to Tb
Calcination temperature °C
1
2:1:1
Maltose
1:1
1000
2
2:1:1
Maltose
1:1
900
3
2:1:1
Maltose
1:1
800
4
2:1:1
Cellulose
1:1
800
5
2:1:1
Date syrup
0.5 gr
800
6
2:1:1
Maltose
1:2
800
7
2:1:1
Maltose
3:2
800
2.3 Photocatalysis tests
The photocatalysis test was performed under UV light to investigate the application of Tb2CoMnO6 nanoparticles in the photocatalysis process. For photocatalysis testing, four solutions of Rhodamine B, Eriochrome Black T, Thymol blue, and Acid red dyes with a concentration of 20 ppm were prepared. The total volume of the dye solution was 100 ml. 0.05 gr of Tb2CoMnO6 nanoparticles were added as a catalyst to the dye solution. The resulting mixture was aerated for 20 min in a light-free medium to equilibrate dye adsorption on the catalyst surface. After that, UV light was applied to the mixture. Sampling was performed at specific times, and after centrifugation and catalyst separation, the adsorption of the solution at λmax of dye was analyzed by UV–Vis spectrometer. The efficiency of the photocatalysis process was calculated as follows: where A0 and At are the absorbance value of dye solution at 0 and t min, respectively (Hassanpour et al., 2021).
3 Results and discussion
In the first step, XRD analysis was used to confirm the formation of Tb2CoMnO6 nanoparticles. However, since these nanoparticles were first synthesized, the XRD pattern recorded in the reference was not found. Hence, the XRD patterns obtained were compared with the existing patterns. A recorded pattern for Gd2CoMnO6 (JCPDS card number: 01-085-0960) was used to confirm the formation of these nanoparticles. As shown in Fig. 1, the pattern obtained for Tb2CoMnO6 nanoparticles is utterly consistent with the reference. This adaptation confirms the orthorhombic phase of Tb2CoMnO6 nanoparticles.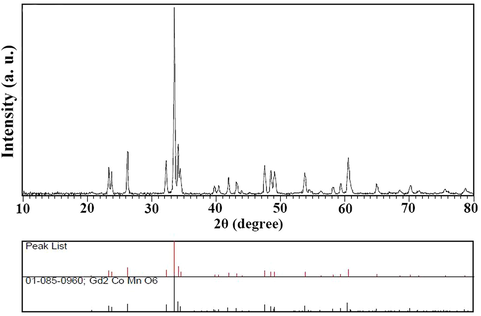
XRD pattern of synthesized Tb2CoMnO6 nanoparticles in the presence of maltose as fuel in comparison with Gd2CoMnO6 reference peak.
The data obtained by X-ray analysis were used to accomplish Rietveld structural refinement to obtain the structural parameters, which was done using FullProf software (Rodríguez-Carvajal, 1993). TbMnO3 structural data was used to perform the Rietveld analysis, which was applied by considering the small difference in the ionic radii of Mn3+ (0.58 Å) and Co3+ (0.54 Å). Also, the occupation of half of the Mn atom position with Co to achieve the Tb2CoMnO6 structure was considered. The result of refined structural parameters in the orthorhombic space group Pbnm were a = 5.312, b = 5.659, c = 7.546 and the reliability factors Rp = 26.7, Rwp = 34, Rexp = 18.20 and χ2 = 3.48. X-ray diffraction Rietveld refinement pattern was shown in Fig. 2. XRD analysis was performed on nanoparticles obtained after calcination at three different temperatures, 1000, 900, and 800 °C. It was found that there is not much difference in the patterns by comparing these three patterns (Fig. 3). So the temperature of 800 °C was chosen as the optimal temperature to continue the synthesis of nanoparticles. The crystallite size of the Tb2CoMnO6 nanoparticles is obtained to be around 37 nm using the Scherrer equation, Dc = Kλ/βCosθ (Hassanpour et al., 2021).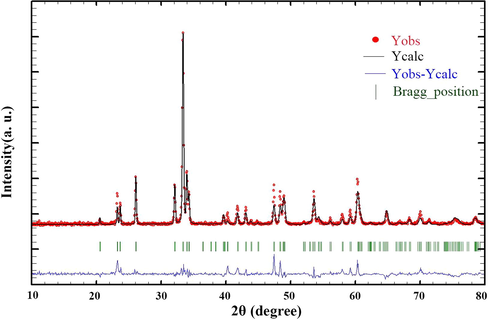
X-ray diffraction Rietveld refinement patterns of Tb2CoMnO6 nanoparticles.
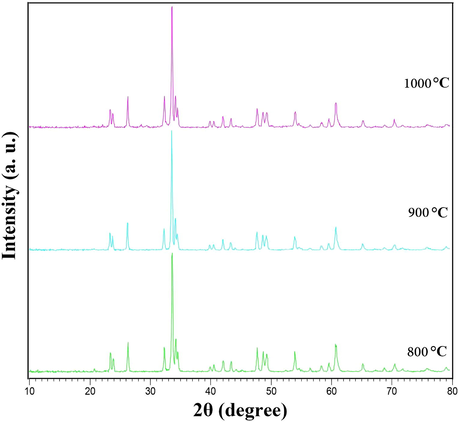
Patterns obtained from XRD analysis of Tb2CoMnO6 nanoparticles prepared in the presence of maltose as fuel at different calcination temperature.
As shown in Table 1, the type and ratio of fuel were investigated to obtain the optimal size and morphology of the nanoparticles. SEM analysis was used to investigate these effects.
The SEM analysis of the effect of different fuels on morphology and particle size was shown in Fig. 4. Three fuels, maltose, cellulose, and Date syrup, were used in this study (Fig. 4 (a), (b), and (c), respectively). The fuel used in the sol–gel method homogenizes the solution by forming a complex with ions and also produces heat, carbon dioxide, and water during the combustion process. Complex formation is actually a type of capping agent that prevents the agglomeration of particles and the formation of by-products. The type of fuel also helps to create better combustion and to form a product with higher purity (Dara et al., 2021; Bhagwat et al., 2019). As is well known, when maltose was used, the particles were smaller in size and wholly monodispersed. However, particles stuck together in the presence of cellulose and Date syrup. According to these images, maltose was selected as the optimal fuel. The study of the effect of fuel on morphology and particle size was continued by changing the ratio of maltose to terbium precursor. As shown in Fig. 5, when a lower fuel ratio was used to perform the reaction (Fig. 5 (a)), the particles coalesced to form elliptical masses. On the other hand, when higher maltose was applied, obtained particles had smaller and almost spherical sizes (Fig. 5 (b)).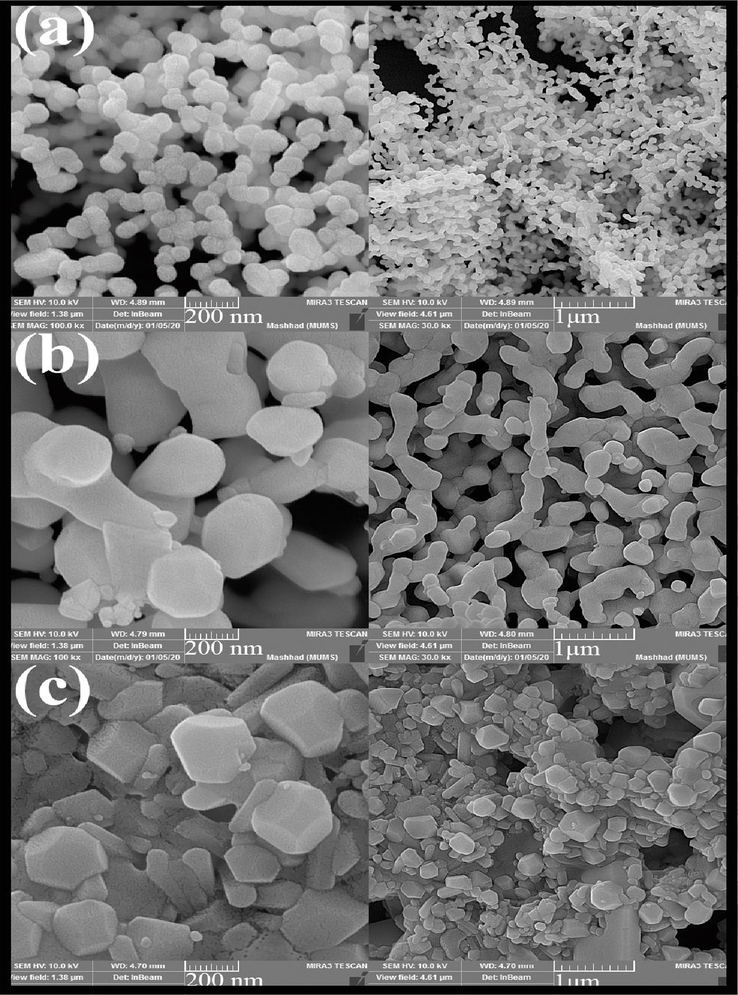
SEM images of Tb2CoMnO6 nanoparticles synthesised in the presence of different fuel (a) Maltose, (b) Cellulose, and (c) Date syrup.
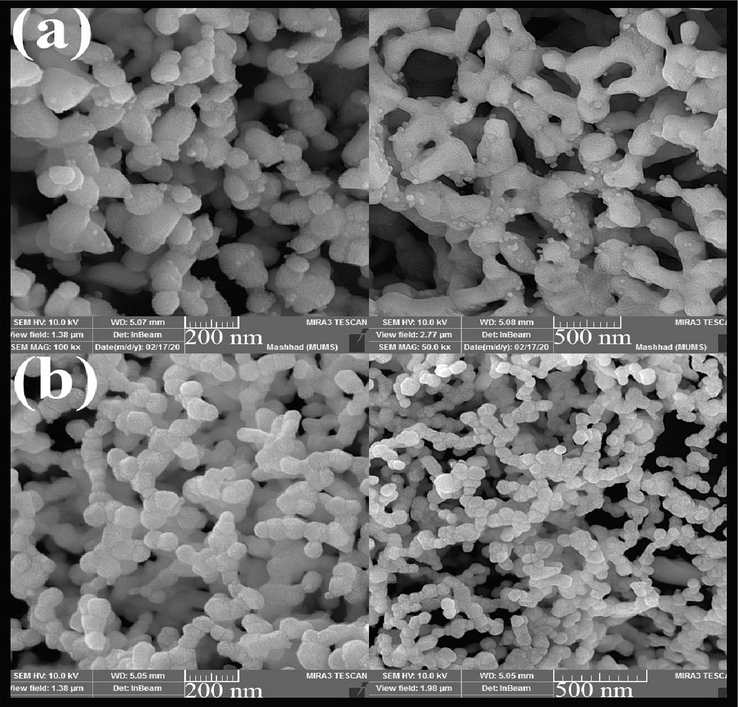
SEM images of Tb2CoMnO6 nanoparticles synthesised in the presence of different ratios of maltose to terbium (a) 1:2 and (b) 3:2.
Finally, by examining the images obtained from SEM and considering the results of XRD analysis, the sample prepared in the presence of maltose as fuel with a ratio equal to terbium was selected as the optimal sample in terms of size and morphology (Sample No. 3) and for further investigations were used.
TEM images were used to investigate the morphology of the particles. TEM images obtained from TFMO nanoparticles of sample S3 were shown in Fig. 6. The nanoparticles obtained in the resulting images showed that the nanoparticles are adhered together and agglomerated. Examination of particle size showed particle size in the range of 30 to 40 nm.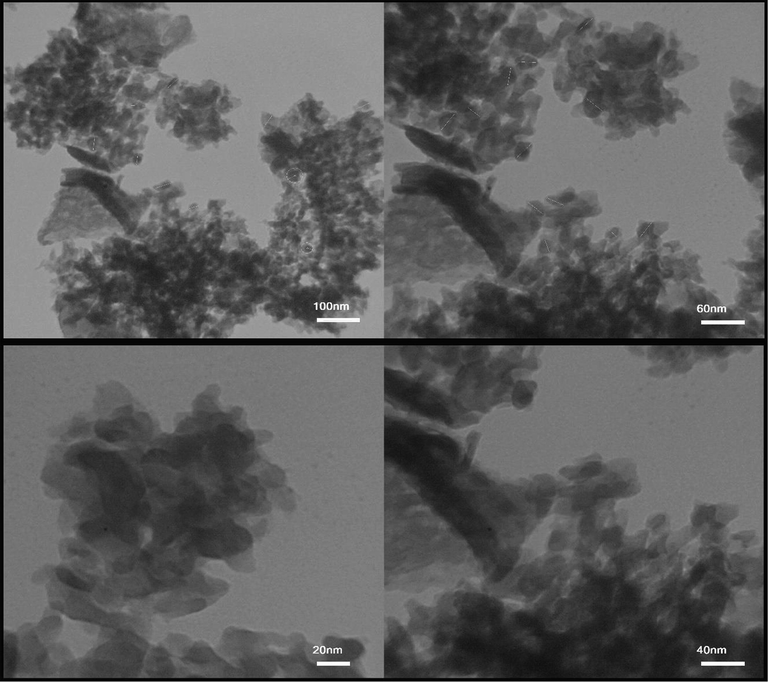
TEM images obtained from Tb2CoMnO6 nanoparticles sample No. 3.
EDS analysis was used to confirm the presence of elements in the composition and confirm the absence of impurities in the synthesized Tb2CoMnO6 nanoparticles. As shown in Fig. 7, Tb, Co, Mn, and O elements in the obtained spectrum were confirmed. Then, FT-IR analysis was performed for the optimal sample to investigate the intermolecular bonds and confirm the complete removal of the organic compounds used in the synthesis steps. Two peaks at 3442 and 1630 cm−1 were observed in the resulting spectrum shown in Fig. 8, which is related to the moisture adsorbed on the surface of the nanoparticles during the sample preparation process for analysis (Hassanpour et al., 2021). The two peaks observed in 595 and 464 cm−1 correspond to the metal–oxygen bonds, which can be related to Mn—O and Co—O, respectively (Dara et al., 2021).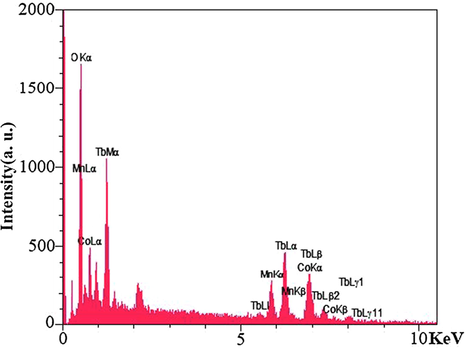
Results of EDS analysis for Tb2CoMnO6 nanoparticles sample No. 3.
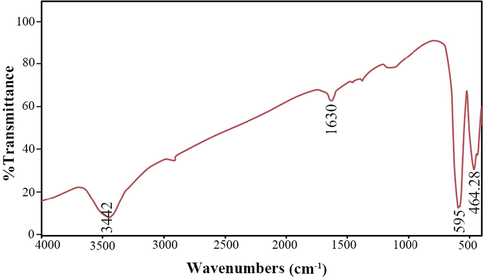
Spectrum obtained from FT-IR analysis Tb2CoMnO6 nanoparticles sample No. 3.
One of the essential features for wastewater treatment using nanoparticles is the accessible collection of nanoparticles after the process (Huang et al., 2015). In fact, the importance of the magnetic properties of nanoparticles is highlighted here. Therefore, the magnetic properties of the synthesized nanoparticles (sample No.3) were investigated using VSM analysis. As shown in Fig. 9, a skinny hysteresis loop showed a coercive field of 200 Oe and remnant magnetization of 0.003 emu.g−1. These negligible values confirm the paramagnetic behavior of the synthesized nanoparticles.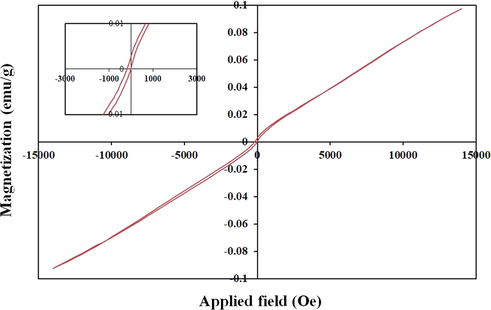
Results of DRS analysis, and the inset shows estimated band-gap of Tb2CoMnO6 nanoparticles sample No. 3.
One of the most important properties in studying the photocatalysis of nanoparticles is their optical properties (Samanta et al., 2018). As mentioned earlier, semiconductor materials with a suitable band-gap are potential options for the photocatalytic process. The value of band-gap plays an essential role in choosing the type of light that is used for irradiation to catalyst. DRS analysis was used to investigate the optical properties of Tb2CoMnO6 nanoparticles (sample No. 3). As shown in Fig. 10, using Tauc’s equation (Tauc et al., 1966), the estimated band-gap for these nanoparticles was calculated to be about 3.2 eV. The literature study confirmed this band-gap range for similar compounds such as 3.28, 3.4, 2.8, and 3.3 eV for Dy2ZnMnO6, Gd2ZnMnO6, Gd2NiMnO6, and Dy2CoMnO6 nanoparticles, respectively (Baladi et al., 2019; Orooji et al., 2020; Mohassel et al., 2020; Valian et al., 2017).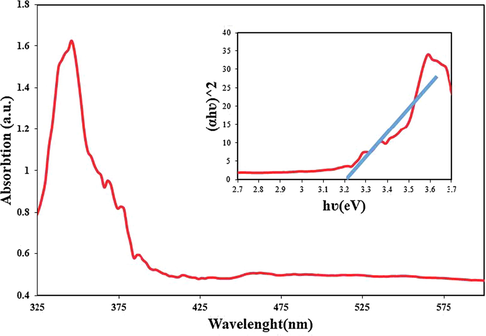
Magnetization versus applied magnetic field at room temperature and the inset shows the magnified hysteresis loop of Tb2CoMnO6 nanoparticles sample No. 3.
The efficiency of Tb2CoMnO6 nanoparticles in the photocatalysis process was investigated for the four organic dyes: Eriochrome black T, Rhodamine B, Thymol blue, and Acid red. The photocatalysis test was performed at ambient temperature under UV light for 90 min. Examination of the results showed a 70% discoloration for Eriochrome Black T in 90 min under UV light. For other dyes, 65, 38, and 22% discoloration was obtained from a solution containing Thymol blue, Acid red, Rhodamine B, respectively (Fig. 11). In general, in explaining the photocatalysis process with the help of semiconductor nanoparticles, the role of the electron-hole pairs caused by light radiation and electron transfer is discussed. Electron-hole pairs formed in the aqueous medium generate free radicals, which can be mentioned as follows (Zhang et al., 2009; Raja et al., 2016; Zhang and Nosaka, 2014):
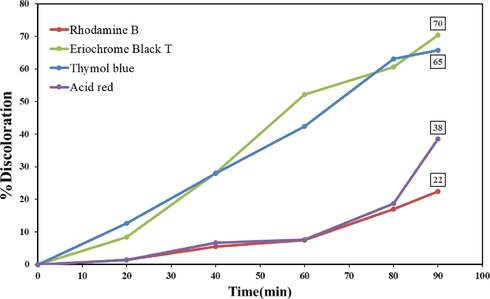
Photocatalytic activity of Tb2CoMnO6 nanoparticles sample No. 3 under UV light.
Which in interaction with organic dyes, degraded them, and discoloration is performed.
Scavengers were used to determine which radical species are effective in color degradation. Various compounds are used to trap free radicals (Schneider et al., 2020; Makama et al., 2020). 1, 4-Benzoquinone, EDTA, and Benzoic acid were used to remove superoxide, h+, and hydroxide radicals, respectively. Therefore, photocatalysis test was performed in the presence of these materials for Eriochrome Black T under the same conditions. As shown in Fig. 12, the results showed that superoxide as a free radical produced in the photocatalysis process plays a significant role in the degradation of dye contamination in the presence of Tb2CoMnO6 nanoparticles. According to the results, the mechanism of the photocatalytic process can be considered as follows:
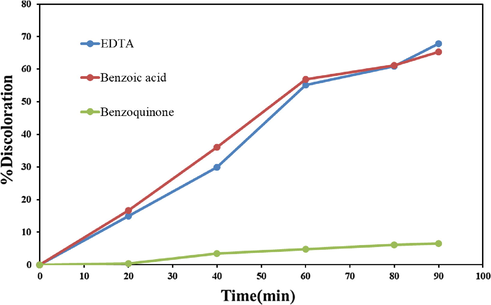
Photocatalytic activity of Tb2CoMnO6 nanoparticles sample No. 3 in the presence of scavengers on discoloration Eriochrome Black T.
4 Conclusion
In summary, Tb2CoMnO6 double perovskite nanoparticles were synthesized by the sol-gel auto-combustion method for the first time. Different fuels were used to investigate the effect of fuel on particle size and morphology. Finally, the nanoparticles synthesized in the presence of maltose were selected as the optimal sample in terms of size and morphology. Various diagnostic and confirmatory analyzes were performed to evaluate the properties of nanoparticles. The estimated band-gap for these nanoparticles was calculated to be about 3.2 eV. Due to the observed appropriate optical properties, these nanoparticles were used as a potential candidate in water treatment in the photocatalytic process with the presence of different organic dyes. The photocatalysis process was performed for the four organic dyes: Eriochrome black T, Rhodamine B, Thymol blue, and Acid red under UV light; the highest discoloration was obtained for Eriochrome black T with 70%.The mechanism of the photocatalytic process was investigated with the help of various scavengers. The results showed that the path of discoloration of contaminants is done with the help of superoxide. In general, the results showed that these materials could be used in the photocatalysis process to remove dye contaminants, although further studies are needed to increase efficiency.
Acknowledgment
The authors are grateful to the council of Iran National Science Foundation; (INSF, 97017837), and the University of Kashan for supporting this work by Grant No (159271/MD3).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng.. 2019;7 103248
- [Google Scholar]
- Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharmaceut. J.. 2019;27:817-829.
- [Google Scholar]
- Sonochemical-assisted synthesis of pure Dy2ZnMnO6 nanoparticles as a novel double perovskite and study of photocatalytic performance for wastewater treatment. Ultrason. Sonochem.. 2019;57:172-184.
- [Google Scholar]
- Sol-gel auto combustion synthesis and characterizations of cobalt ferrite nanoparticles: Different fuels approach. Mater. Sci. Eng., B. 2019;248:114388
- [Google Scholar]
- Perovskite-type CaMnO3 anode material for highly efficient and stable lithium ion storage. J. Colloid Interface Sci.. 2021;584:698-705.
- [Google Scholar]
- The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res.. 2019;52:596-604.
- [Google Scholar]
- Green sol–gel auto combustion synthesis and characterization of double perovskite Tb2ZnMnO6 nanoparticles and a brief study of photocatalytic activity. RSC Adv.. 2021;11(14):8228-8238.
- [Google Scholar]
- Ti-substituted LaFeO3 perovskite as photoassisted CWPO catalyst for water treatment. Appl. Catal. B: Environ.. 2019;248:120-128.
- [Google Scholar]
- Perovskite nanomaterials as optical and electrochemical sensors. Inorg. Chem. Front.. 2020;7(14):2702-2725.
- [Google Scholar]
- Progress in manufacture and properties of construction materials incorporating water treatment sludge: A review. Resour. Conserv. Recycl.. 2019;145:148-159.
- [Google Scholar]
- Sol-gel synthesis and characterization of Co3 O4/CeO2 nanocomposites and its application for photocatalytic discoloration of organic dye from aqueous solutions. Environ. Sci. Pollut. Res.. 2021;28(6):7001-7015.
- [Google Scholar]
- Toxicity evaluation and preparation of CoWO4 nanoparticles towards microalga Dunaliella salina. Environ. Sci. Pollut. Res.. 2021;28(27):36314-36325.
- [Google Scholar]
- Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf., A. 2015;478:71-80.
- [Google Scholar]
- A review of recent advances and future challenges in freshwater salinization. Limnetica. 2020;39:185-211.
- [Google Scholar]
- An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res.. 2020;27:2522-2565.
- [Google Scholar]
- Influence of parameters and radical scavengers on the visible-light-induced degradation of ciprofloxacin in ZnO/SnS2 nanocomposite suspension: Identification of transformation products. Chemosphere. 2020;253:126689
- [Google Scholar]
- Pechini synthesis using propylene glycol and various acid as stabilizing agents and characterization of Gd2NiMnO6 ceramic nanostructures with good photocatalytic properties for removal of organic dyes in water. J. Mater. Res. Technol.. 2020;9(2):1720-1733.
- [Google Scholar]
- Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater.. 2020;383:121125
- [Google Scholar]
- Climate change in northern Patagonia: critical decrease in water resources. Theor. Appl. Climatol. 2020:1-16.
- [Google Scholar]
- Gd2ZnMnO6/ZnO nanocomposites: Green sol-gel auto-combustion synthesis, characterization and photocatalytic degradation of different dye pollutants in water. J. Alloys Compounds 2020 155240
- [Google Scholar]
- A study on the free radical generation and photocatalytic yield in extended surfaces of visible light active TiO2 compounds. Sol. Energy Mater. Sol. Cells. 2016;152:125-132.
- [Google Scholar]
- Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 1993;192:55-69.
- [Google Scholar]
- Optical properties and enhanced photocatalytic activity of Mg-doped ZnO nanoparticles. Physica E. 2018;104:254-260.
- [Google Scholar]
- Lead free double perovskite oxides Ln2NiMnO6 (Ln= La, Eu, Dy, Lu), a new promising material for photovoltaic application. Mater. Sci. Eng., B. 2017;226:10-17.
- [Google Scholar]
- Use of scavenger agents in heterogeneous photocatalysis: truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys.. 2020;22(27):15723-15733.
- [Google Scholar]
- La2MnTiO6 double perovskite nanostructures as highly efficient visible light photocatalysts. New J. Chem.. 2020;44(1):231-238.
- [Google Scholar]
- Vibrational properties and infrared dielectric features of Gd2CoMnO6 and Y2CoMnO6 double perovskites. Ceram. Int.. 2019;45(4):4756-4762.
- [Google Scholar]
- Structure, electronic structure, optical and magnetic studies of double perovskite Gd2MnFeO6 nanoparticles: First principle and experimental studies. Mater. Today Commun.. 2020;25 101603
- [Google Scholar]
- Optical properties and electronic structure of amorphous Germanium. Phys. Status Solidi (b). 1966;15(2):627-637.
- [Google Scholar]
- Urchin-like Dy2CoMnO6 double perovskite nanostructures: new simple fabrication and investigation of their photocatalytic properties. J. Mater. Sci.: Mater. Electron.. 2017;28:12440-12447.
- [Google Scholar]
- Double-perovskite magnetic La2NiMnO6 nanoparticles for adsorption of bovine serum albumin applications. Nanoscale Res. Lett.. 2013;8:1-4.
- [Google Scholar]
- The photostability of wool doped with photocatalytic titanium dioxide nanoparticles. Polym. Degrad. Stab.. 2009;94:278-283.
- [Google Scholar]
- Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C. 2014;118:10824-10832.
- [Google Scholar]
- Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol., Monitoring Manage.. 2019;12 100255
- [Google Scholar]







