Translate this page into:
The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities
⁎Corresponding authors. wangcaixia1111@163.com (Caixia Wang), xucuilian666@126.com (Cuilian Xu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A series of novel coumarin thiazoles containing trifluoromethyl group 3a-3l were prepared through a one-pot reaction using 3-(trifluoroacetyl)coumarin as a starting material in solvent-free conditions. Their structures were confirmed using IR, 1H NMR, 13C NMR, HRMS and X-ray single crystal diffraction, and their antifungal activity against Fusarium. moniliforme(F. moniliforme), Fusarium. graminearum (F. graminearum), and Curvularia. lunata (C. lunata) was evaluated. Among the synthesized compounds, compound 3f showed the highest inhibitor rate of 74% at 0.5 mg/mL against F. moniliforme, and compound 3g exhibited the highest inhibitor rates of 89% and 93.4% at 0.5 mg/mL against F. graminearum and C. lunata, respectively. The introduction of trifluoromethyl group greatly improved the antifungal activity of counterpart 3g compared to the compound 4.
Keywords
Coumarin thiazoles
Trifluoromethyl
Solvent-free
Fungicidal activity
1 Introduction
Fungal disease in agriculture leads to great annual economic losses to farmers worldwide. For example, F. graminearum causes wheat head blight in wheat-growing regions (Duan et al., 2019), and F. moniliforme generally results in corn ear rot, which is a destructive disease that decreases corn yield (Fu et al., 2015). The excessive use of antifungal agents increased the resistance of fungi to biocides. The development of new lead antifungal compounds is an urgent and arduous task for agrochemical researchers.
Coumarins are a class of natural heterocycle products that contain oxygen with a benzopyrone ring. Moreover, thiazoles are also very important five-membered heterocyclic compounds with nitrogen in nature. Coumarins and thiazoles have many common biological activities in medicinal and pharmaceutical areas such as antifungal (Elias et al., 2019, Yan et al., 2019), anticancer (Abdo et al., 2019; Morsy et al., 2017), antibacterial (Wang et al., 2019; Grybaite et al., 2019), and antiviral properties (Shen et al., 2018; Sokolova et al., 2018).
Hybrid molecules of two or more existing pharmacophores are attractive because of their multiple mechanisms of action (Feng et al., 2020). Some previous studies demonstrated that coumarin-thiazole hybrids showed significant antibacterial (Liu et al., 2020), antidiabetic (Tegginamath et al., 2016), anti-inflammatory (Ramagiri et al., 2015), antiviral (Osman et al., 2018) and anticancer (Ayati et al., 2018) biological activities. The hybridization of coumarin with thiazole also provides opportunities for the development of novel antifungal compounds (Abdel-Aziem, 2015, Chandak et al., 2014). Yuan et al. synthesized coumarin-modified thiazole with good antifungal activities via the condensation of aminothiourea with 3-acetylcoumarin intermediates (Yuan et al., 2011).
Notably, the introduction of a trifluoromethyl group in a target molecule improved the properties of compounds, such as electrostatic interactions, lipid solubility, and oxidative thermal stability (Zhukovskaya et al., 2018). Some trifluoromethylated heterocyclic compounds showed prominent biological activities in pharmaceutical research fields (Aggarwal et al., 2013, Chen et al., 2017, Kratky et al., 2020, Tan et al., 2020, Tempesti et al., 2011, Yang et al., 2018) (Fig. 1).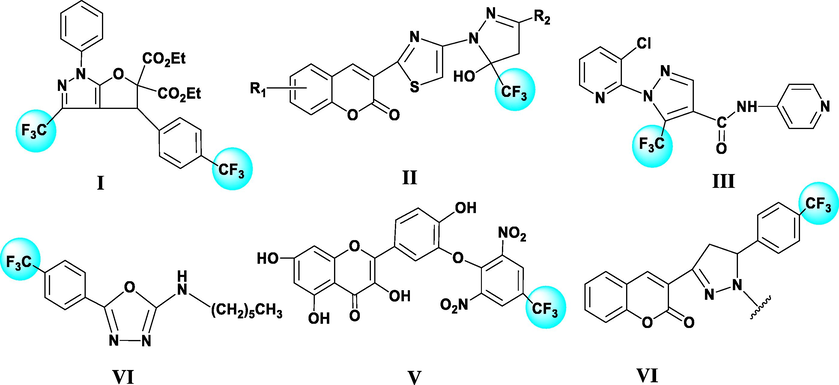
Some bioactive heterocyclic compounds containing a trifluoromethyl moiety.
Our group focused on the design and synthesis of novel coumarin derivatives with biological activities (Shi et al., 2020). With the inspiration of trifluoromethylated heterocyclic compounds having higher activities, we designed and prepared a series of novel coumarin thiazoles containing a trifluoromethyl moiety using an efficient, solvent-free, one-pot method and evaluated the antifungal activities of these compounds (Fig. 2).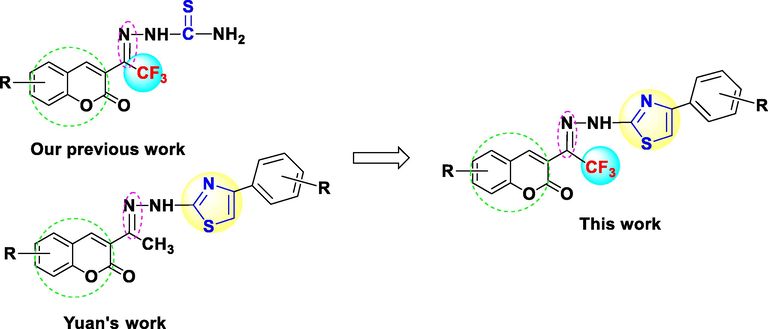
Strategy for the synthesis of coumarin with thiazole and trifluoromethyl moieties.
2 Materials and methods
2.1 Chemicals and instruments
3-(Trifluoroacetyl)coumarin 1 was synthesized according our reported procedure (Yang et al., 2015).
Coumarin thiazole 4(6-chloro-3-((E)-1-(((Z)-3-methyl-4-phenylthiazol-2(3H)-ylidene)hydrazono)ethyl)-2H-chromen-2-one) with methyl moiety instead of trifluoromethyl moiety was obtained and confirmed according to Yuan’s method (Yuan et al., 2011). Other used chemicals were purchased from commercial sources and used without further purification. 1H NMR and 13C NMR spectra were obtained using a Bruker DPX-400 spectrometer in CDCl3 or DMSO solution with TMS as an internal standard. HR-MS(ESI) spectra were performed using a Waters Q-Tof MicroTM instrument, and X-rays were measured at 293 K on a Rigaku RAXIAS-IV type diffractometer. Three crop-threatening pathogenic fungi (F. moniliforme, F. graminearum, and C. lunata) were obtained from College of Plant Protection in Henan Agricultural University. Most reaction yields, except compound 3a, were not optimized.
CCDC 1525208 contains the supplementary crystallographic data of compound 3 in this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data-request/cif.
2.1.1 General procedure for the preparation of compounds 3a-3l
3-(Trifluoroacetyl)coumarin 1a (5.0 mmol) and 6.0 mmol of thiosemicarbazide were added to a solid powder of silica gel-supported TsOH (0.5 mmol) in a mortar. These substances were mixed well, and the mixture was heated in an oven. Once the reaction was completed according to TLC or HPLC, 5.0 mmol of 2-bromoacetophenone was blended with the above mixture, and heating was continued until compound 2a was nearly disappeared. After the mixture cooled to room temperature, the product was extracted with 3 × 10 mL of dichloromethane. The combined extract was concentrated in a vacuum to obtain crude products, and the crude products was purified via recrystallization when necessary to give a yellow solid 3a.
The preparation of compounds 3b-3l was the same as compound 3a.
2.1.2 Compounds data
(E)-3-(2,2,2-trifluoro-1-(2-(4-phenylthiazol-2-yl)hydrazono)ethyl)–2H-chromen-2-one (3a):
Yellow powder, m.p.159.0–161.7℃. IR (v, cm−1, KBr): 3436 (m), 1720 (s), 1614 (s), 1571 (w), 1543 (w), 1451 (w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 7.30 (t, J = 8 Hz, 1H, Ar-H), 7.39 (t, J = 8 Hz, 2H, Ar-H), 7.46 (t, J = 8 Hz, 1H, Ar-H), 7.54 (d, J = 6 Hz, 2H, Ar-H), 7.77 (t, J = 8 Hz,1H, Ar-H), 7.85–7.81 (m, 3H, Ar-H), 8.43 (s, 1H, CH=), 12.44 (s, 1H, NH). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 106.09 (CH), 116.33 (CH), 116.64 (CH), 118.38 (C), 120.78 (q, J = 271 Hz, CF3), 127.62 (q, J = 34 Hz, C-CF3), 124.87 (2C, CH), 125.49 (CH), 127.79 (CH), 128.66 (2C, CH), 129.40 (CH), 133.56 (CH), 134.25 (C), 146.96 (CH), 150.87 (C), 153.98 (C), 157.39 (C=O), 167.25 (C=N); HRMS (ESI): calcd for C20H13F3N3O2S: (M+H+) 416.0681; found: 416.0677.
3-((1E)-2,2,2-trifluoro-1-((3-methyl-4-phenylthiazol-2(3H)-ylidene)hydrazono)ethyl)-2H-chromen-2-one (3b):
Yellow powder; m.p.:183.0–183.4℃; IR (v, cm−1, KBr): 3447 (m), 1735 (s), 1607 (m), 1506 (s), 1423 (m); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.20 (s, 3H, CH3), 6.71 (s, 1H, -S-CH=), 7.42–7.50 (m, 7H, Ar-H), 7.70 (s, 1H, Ar-H), 7.84 (d, J = 8 Hz, 1H, Ar-H), 8.30 (s, 1H, CH=). 13C NMR (100 MHz, DMSO -d6, δ, ppm): 40.45 (CH3), 109.19 (CH), 122.99 (C), 124.65 (CH), 125.88 (C), 127.87 (q, J = 271 Hz, CF3), 131.63 (CH), 135.46 (2C, CH), 135.57 (CH), 135.83 (CH), 136.13 (CH), 136.39 (CH), 139.70 (C), 142.71 (q, J = 33 Hz, C-CF3), 147.63 (CH), 151.20 (C), 151.31 (C),C), 160.04 (C), 163.46 (C=N), 181.24 (C=O); HRMS (ESI) : calcd for C21H15F3N3O2S: (M + H+) 430.0837; found: 430.0835.
3-((1E)-1-((3,4-diphenylthiazol-2(3H)-ylidene)hydrazono)-2,2,2-trifluoroethyl)–2H-chromen-2-one (3c):
Yellow powder; IR (v, cm−1, KBr): 3444 (m), 3098 (m), 1734 (s), 1608 (m), 1502 (s), 1490 (s),1454 (m) ; 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 6.91 (s, 1H, CH = ),7.07–7.16 (m, 7H, Ar-H), 7.23–7.28 (m, 3H, Ar-H), 7.37–7.45 (m, 2H, Ar-H), 7.66–7.73 (m, 2H, Ar-H), 8.16 (s, 1H, CH = ). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 104.13 (CH), 116.49 (C), 118.22 (CH), 121.43 (q, J = 270.8 Hz, CF3), 120.14 (C), 125.33 (CH), 128.49 (CH), 128.80 (CH), 128.98 (CH), 129.29 (CH), 129.65 (CH), 133.57 (C), 136.88 (CH=), 138.66 (q, J = 33 Hz, C-CF3), 140.46 (C), 145.51 (C), 153.67 (C), 157.44 (C-S), 174.48 (C=O); HRMS (ESI): calcd for C26H17F3N3O2S: (M+H+) 492.0994; found: 492.0986.
3-((E)-1-(((Z)-3-(4-chlorophenyl)-4-phenylthiazol-2(3H)-ylidene)hydrazono)-2,2,2-trifluoroethyl)-2H-chromen-2-one (3d):
White powder; IR (v, cm−1 KBr): 3429 (s), 1734 (m), 1667 (s), 1608 (s), 1504 (s), 1455 (m), 1402 (w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 6.31 (s, 1H, CH=), 7.01–7.07 (m, 5H, Ar-H), 7.20–7.29 (m, 5H, Ar-H), 7.37 (q, J = 8.0 Hz, 2H, Ar-H), 7.56 (t, J = 6.0 Hz, 1H, Ar-H), 7.64 (s, 1H, CH = ). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 103.10 (CH), 116.52 (C), 118.11 (CH), 121.06 (q, J = 272.0 Hz, CF3), 120.69 (C), 124.53 (CH), 128.42 (C), 128.45 (CH), 128.56 (CH), 128.65 (CH), 128.97 (CH), 129.37 (CH), 130.05 (CH), 132.47 (CH), 133.51 (C), 135.26 (CH), 139.51 (q, J = 33.9 Hz, C-CF3), 140.11 (C), 142.82 (C), 153.88 (C), 157.39 (C=N), 174.05 (C=O); HRMS (ESI): calcd for C26H15ClF3N3O2S: (M+H+) 526.0604; found: 526.0595.
(E)-6-methyl-3-(2,2,2-trifluoro-1-(2-(4-phenylthiazol-2-yl)hydrazono)ethyl)–2H-chromen-2-one(3e):
Yellow powder; m.p.: 210.5–212.8℃; IR (v, cm−1 KBr): 3428(w), 3060(w), 2923(w), 1725 (s), 1619 (m), 1573 (s), 1482 (m); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 2.41 (s, 3H, CH3), 7.31 (t, J = 8 Hz, 1H, CH), 7.41 (q, 3H, Ar-H), 7.49 (s, 1H, Ar-H), 7.57 (d, J = 8 Hz, 1H, Ar-H), 7.62 (s, 1H, Ar-H), 7.84 (d, J = 8 Hz, 2H, Ar-H), 8.33 (s, 1H, CH=), 12.45 (s, 1H, NH). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 20.64 (CH3), 106.37 (CH), 116.55 (CH), 117.23(C), 118.61 (C), 121.31 (q, J = 271.1 Hz, CF3), 126.00 (CH), 128.28 (CH), 129.13 (CH), 129.40 (CH), 134.64 (CH), 134.82 (C), 147.18 (C=C), 151.22 (C), 152.65 (C), 158.04 (C=O), 168.06 (C=N); HRMS (ESI): calcd for C21H15F3N3O2S: (M+H+) 430.0837; found:430.0835.
(E)-7-methoxy-3-(2,2,2-trifluoro-1-(2-(4-phenylthiazol-2-yl)hydrazono)ethyl)–2H-chromen-2-one (3f):
Yellow powder; m.p.:190.1–191.9℃; IR (v, cm−1 KBr): 3435 (s), 2967 (w), 1708 (s), 1604 (s), 1563 (w), 1507 (w), 1368 (w), 1313 (w), 1247 (w), 1234 (w), 1191 (s), 1160 (s), 1125 (s), 1029 (s), 1005 (s), 682(s); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.91 (s, 3H, CH3), 7.055 (dd, 1H, J = 2 Hz, J = 1 Hz, Ar-H), 7.149 (d, J = 2 Hz, 1H,), 7.30 (t, J = 1.9 Hz,1H, Ar-H), 7.40 (t, J = 1.8 Hz, 2H, Ar-H), 7.50 (s, 1H,Ar-H), 7.76 (s, J = 2.1 Hz,1H, CH=), 7.83 (s, J = 2.1 Hz, 2H, Ar-H), 8.31 (s, 1H, CH=). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 56.50 (CH3), 98.10 (CH), 99.54 (CH), 105.01 (CH), 105.74 (C), 126.78(CH), 129.70 (CH), 130.69 (C), 130.89 (CH), 132.72 (C), 132.90 (CH), 152.96 (CH), 155.60 (C), 159.77 (C), 160.59 (C), 163.44 (C=N), 182.24 (C=O); HRMS (ESI) : calcd for C21H15F3N3O3S: (M+H+) 446.0786; found: 446.0787.
6-chloro-3-((E)-2,2,2-trifluoro-1-(((Z)-3-methyl-4-phenylthiazol-2(3H)-ylidene)hydrazono)ethyl)–2H-chromen-2-one (3 g):
Yellow powder; m.p.:185.4–188.3℃; IR (v, cm−1 KBr): 3424 (w), 1759 (s), 1580 (w), 1495 (s), 1420 (w), 1443 (w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm):3.25 (s, 3H, CH3), 6.16(s, 1H, CH), 7.26–7.33 (m, 3H, Ar-H), 7.45 (t, 3H, Ar-H), 7.52 (s, 2H, Ar-H), 7.74 (s, 1H, CH=). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 33.93 (CH3), 102.22 (CH), 118.21 (C), 119.45 (CH), 121.27 (q, J = 271 Hz, CF3), 122.27 (C), 127.61 (CH), 128.90 (CH), 128.94 (CH), 129.60 (CH), 129.86 (CH), 130.18 (C), 132.22 (C), 136.38 (q, J = 34 Hz, C-CF3), 141.21 (CH=), 141.79 (C), 152.48 (C), 156.67 (C=N), 175.42 (C=O); HRMS (ESI): calcd for C21H13ClF3N3O2S: (M+H+) 464.0447. found: 464.0448.
6-bromo-3-((E)-2,2,2-trifluoro-1-(((Z)-3-methyl-4-phenylthiazol-2(3H)-ylidene)hydrazono)ethyl)–2H-chromen-2-one (3h)
Yellow powder; m.p.:193.2–195.5℃; IR (v, cm−1 KBr): 3282 (m), 2983 (w), 1707 (s), 1601 (s), 1470 (m); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.21(s, 3H, CH3), 6.72(s, 1H, CH-S), 7.46 (d, J = 8.0, 2.0 Hz, 1H, Ar-H), 7.50 (s, 5H, Ar-H), 7.845 (dd, J = 8.0 Hz, 1H, Ar-H), 8.095 (d, J = 4.0 Hz, 1H, Ar-H), 8.25(s, 1H, CH = ). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 34.33 (CH3), 103.18 (CH), 117.00 (C), 119.15 (CH), 120.32 (C), 120.81 (C), 129.30 (CH), 129.43 (CH), 129.99 (CH), 130.20(CH), 131.64 (CH=), 135.84 (CH), 141.52 (CH), 143.84 (C), 152.95 (C), 156.82 (C=N), 175.21 (C=O); HRMS (ESI): calcd for C21H14BrF3N3O2S: (M+H+) 507.9942. found: 507.9928.
(E)-3-(1-(2-(4-(4-bromophenyl)thiazol-2-yl)hydrazono)-2,2,2-trifluoroethyl)–2H-chromen-2-one(3i)
Yellow powder; m.p.: 160.0–162.5℃; IR (v, cm-1 KBr): 3421 (w),1722(m), 1608 (s), 1568 (w), 1490(w), 1455(w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 6.54 (s, 1H, CH=), 7.26–7.32 (m, 2H, Ar-H), 7.37 (s, 1H, CH=), 7.39 (s, 1H, Ar-H), 7.60 (td, J = 8.0, 4.0 Hz, 1H, Ar-H), 7.80 (s, 1H, Ar-H). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 105.56 (CH=), 115.46 (CH), 116.60 (C), 117.62 (C), 120.51 (q, J = 272 Hz, CF3), 125.06 (CH), 127.02 (CH), 129.34 (CH), 131.81 (CH), 132.11 (CH), 133.82 (C), 147.26 (CH=), 149.32 (C=), 154.24 (C), 156.67 (C=O), 168.22 (C=N); HRMS (ESI): calcd for C20H11BrF3N3O2S: (M+H+) 493.9780, found: 493.9575.
(E)-3-(2,2,2-trifluoro-1-(2-(4-(4-methoxyphenyl)thiazol-2-yl)hydrazono)ethyl)–2H-chromen-2-one(3j)
Yellow powder; m.p.: 155.0–157.0℃; IR (v, cm−1 KBr): 3423 (w), 1609 (s), 1489 (w), 1385(w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.76 (s, 3H, CH3), 6.94 (d, J = 8.0 Hz, 1H, CH=), 7.10 (t, J = 4.0 Hz, 1H, CH=), 7.46 (t, J = 8.0 Hz, 2H, Ar-H), 7.53 (d, J = 8.0 Hz, 1H, Ar-H), 7.74 (d, J = 8.0 Hz, 2H, Ar-H), 7.85 (t, J = 8.0, 4.0 Hz, 1H, Ar-H), 8.41 (s, 1H, Ar-H), 12.38 (s, 1H, N-H). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 55.4 (CH3),102.71 (CH=), 114.15 (CH), 115.71 (C), 117.79 (C), 120.59 (q, J = 272 Hz, CF3), 124.80 (C), 125.93 (CH), 126.85 (CH), 129.24 (CH), 130.38 (q, J = 36 Hz, C-CF3), 133.48 (C), 147.30 (CH=), 149.81 (C),154.22 (C), 156.61 (C=O), 159.72 (C-O), 168.28 (C=N); HRMS (ESI): calcd for C21H15F3N3O3S: (M + H+) 446.0786. found: 446.0703.
((E)-2,2,2-trifluoro-1-(((Z)-3-methyl-4-phenylthiazol-2(3H)-ylidene)hydrazono)ethyl)–2H-benzo[g]chromen-2-one(3 k)
Yellow powder; m.p. 267.6–268.8℃; IR (v, cm−1 KBr): 3424 (s), 1721 (s), 1613 (s), 1571 (w), 1503 (w), 1422 (w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.17(s, 3H, CH3), 6.08 (s, 1H, CH=), 7.187 (s, 1H,Ar-H), 7.25(t, J = 3.6 Hz, 1H, Ar-H), 7.36(t, J = 11 Hz, 2H, Ar-H), 7.42 (d, J = 8.0 Hz, 1H, Ar-H), 7.51 (t, J = 8.0 Hz, 1H, Ar-H), 7.63 (t, J = 8.0 Hz, 1H, Ar-H), 7.85 (d, J = 8.0 Hz, 1H, Ar-H), 7.96 (d, J = 9.2 Hz, 1H, Ar-H), 8.18 (d, J = 8.0 Hz, 1H, Ar-H), 8.55 (s, 1H, CH=). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 32.88(CH3), 101.06 (CH=), 111.58 (C), 115.88 (CH), 118.70 (CH), 120.43 (CH), 125.16 (CH), 127.49 (C), 127.87 (CH), 128.06 (CH), 128.19 (CH), 128.50 (CH), 129.26 (C), 129.29 (C), 132.74 (CH), 138.05 (C), 154.22 (C), 140.12 (C), 153.23 (C=N), 166.36 (C=O), 174.27 (C=N); HRMS (ESI): calcd for C25H17F3N3O2S: (M + H+) 480.0994. found: 480.0996.
3-((E)-2,2,2-trifluoro-1-(((Z)-4-(4-methoxyphenyl)-3-methylthiazol-2(3H)-ylidene)hydrazono)ethyl)–2H-benzo[g]chromen-2-one(3l)
Yellow powder; IR (v, cm−1 KBr): 3443(s), 1707(s),1609 (s), 1593 (s), 1567 (m), 1522 (w), 1454 (m),1411 (w); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 3.26(s, 3H, CH3), 3.86(s, 3H, CH3), 6.13 (s, 1H, CH = ), 6.97 (d, 1H, Ar-H), 7.26(t, J = 8.0 Hz, 2H, Ar-H), 7.28(s, 1H, Ar-H), 7.52 (d, J = 8.0 Hz, 1H, Ar-H), 7.61 (t, J = 8.0 Hz, 1H, Ar-H), 7.73 (t, J = 8.0 Hz, 1H, Ar-H), 7.95 (d, J = 8.0 Hz, 1H, Ar-H), 8.05 (d, J = 8.0 Hz, 1H, Ar-H), 8.28 (d, J = 8.0 Hz, 1H, Ar-H), 8.65 (s, 1H, CH = ).13C NMR (100 MHz, DMSO‑d6, δ, ppm): 33.90 (CH3), 55.41 (CH3), 101.49 (CH=), 112.62 (C),114.28 (CH), 116.91 (CH), 119.63 (C), 121.48 (CH), 122.42 (C), 126.20 (CH), 128.52 (CH), 129.08 (C), 129.20 (=CH), 130.32 (2CH), 133.80 (C), 139.16 (C), 141.00 (C), 154.10 (C=O), 157.44 (C=O), 160.48 (C-O), 175.18 (C=N); HRMS (ESI): calcd for C26H19F3N3O3S: (M + H+) 510.1099. found: 510.1095.
2.2 In vitro antifungal assay
The antifungal activity of compounds 3a-3l and 4 without trifluoromethyl group was performed based on the method of Tan et al (Tan et al., 2018). The synthesized compounds were dissolved in a 20% DMSO water solution. The solution of each compound was added to sterilized potato dextrose agar to produce a final concentration of 500 μg/mL. After the mixture was cooled, the mycelium of fungi were transferred to the test plate and incubated at 25 ℃ for 3–7 days. When the mycelium of fungi reached the edges of the control plate (without the added samples), the inhibitory index was calculated as follows: Inhibitory index (%)=(1 − Da/Db), where Da is the diameter of the growth zone in the test plate, and Db is the diameter of the growth zone in the control plate. Each experiment was performed three times, and the data were averaged.
3 Results and discussion
3.1 Synthesis
2-(Coumarin-3-yl)trifluoroethylidene-modified thiazoles 3 were prepared via the synthetic pathways shown in Scheme 1. We performed the reaction in two steps under solvent-free and one-pot conditions. The 3-(trifluoroacetyl)coumarin 1 was condensed with thiosemicarbazide to yield coumarin-modified thiosemicarbazone 2 under the catalysis of TsOH using silica gel as a supporting material. HPLC monitoring revealed that, upon consumption of the 3-(trifluoroacetyl)coumarin 1 after approximately 1–3 h, the unisolated compound 2 was treated with 2-bromoacetophenone to produce coumarin-modified thiazole 3 following the well-known Hantzsch’s thiazole synthesis. The formation of thiazole in silica gel was indicated by the yellow appearance of the reaction mixture and was completed in 1–2 h (monitored by HPLC).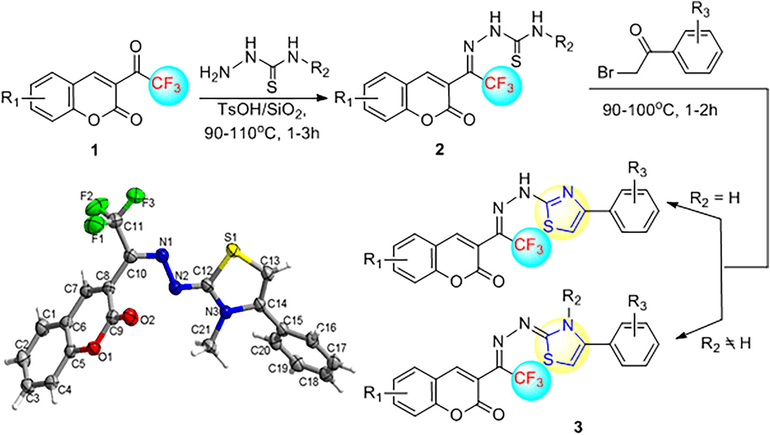
Synthesis of 2-(coumarin-3-yl)trifluoroethylidene-modified thiazole 3.
Using the solvent-free, one-pot method, we synthesized a series of novel 2-(coumarin-3-yl)trifluoroethylidene-modified thiazoles, 3a-3l, as shown in Table 1. During the experimental process, we found that coumarin-bearing electron-withdrawing substituents, such as chlorine and bromine, were unfavorable to Hantzsch’s thiazole synthesis. We tried many conditions, but we could not obtain two products, where R1 = 6-Cl or 6-Br and R2, R3 = H. When thiosemicarbazide with R2 = methyl was used instead, we successfully synthesized the compounds 3g and 3h. The results showed that the coumarin and thiosemicarbazide electron-donating groups promoted the formation of the thiazole cycle. The 2-bromoacetophenone group had no significant impact on the cyclization reaction of thiazole. Many 2-bromoacetophenone-bearing electron-donating and electron-withdrawing substituents proceeded smoothly in this one-pot reaction to provide the title compound 3 in moderate yields. Reaction conditions: esters 1 (5 mmol), TsOH/SiO2 (10 mol %), thiosemicarbazides (6 mmol), 2-bromoacetophenone (5 mmol) under solvent-free one-pot conditions.
Product
R1
R2
R3
Time (temperature)/h (℃)
Yield/% a
1st step
2nd step
3a
H
–
H
1.5(100)
1(100)
65
3b
H
CH3
H
2.5(90)
1.5(90)
60
3c
H
Phenyl
H
2.5(100)
1.5(100)
61
3d
H
4-Chlorophenyl
H
2(90)
0.8(90)
66
3e
6-CH3
–
H
3(90)
0.6(90)
63
3f
7-OCH3
–
H
1.5(100)
1(100)
72
3g
6-Cl
CH3
H
2(100)
1(100)
61
3h
6-Br
CH3
H
1.5(100)
1.5(100)
54
3i
H
–
4-Br
1.5(100)
1(90)
69
3j
H
–
4-OCH3
1.5(100)
1(90)
47
3k
6,7-Benzo
CH3
H
3(110)
0.5(90)
71
3l
6,7-Benzo
CH3
4-OCH3
3(110)
0.5(90)
55
The structures of the synthesized compounds 3a-3l were established using spectral data, such as IR, 1H NMR, 13C NMR and HRMS analyses. The 1H NMR spectra of 3a showed one singlet of one proton intensity that appeared at approximately δ 12.4 ppm due to H at the nitrogen connected with thiazole. Nevertheless, in the 1H NMR spectra of 3b, The signal of H close to the nitrogen could not be observed. The 13C NMR spectra of 3a confirmed the presence of the thiazole ring by exhibiting signals at δ 167, 147 and 106 ppm attributing to C2, C4 and C5 of the thiazole ring, respectively. Owing to the existance of methyl group, C2 formed a double bond with nitrogen outside the thiazole ring. The nitrogen is closer to the electrondrawing trifluoromethyl group than that of thiazole ring, which resulted in the signal shift of C2, C4 and C5 to 181, 148 and 109 ppm. These signals indicated that the thiazole ring in 3a was different from that in 3b.
3.2 In vitro antifungal activity
The target compounds 3a-3l were screened for preliminary antifungal activity against F. moniliforme, F. graminearum and C. lunata at a concentration of 0.5 mg/mL using the mycelial growth rate method. Compound 4 and standard triadimefon and were also tested under similar conditions for comparison.
The antifungal results of the fluorinated coumarin thiazoles (3a-3l) against F. moniliforme are shown in Fig. 3. The inhibitor rate of compound 3a was 28.27% at 0.5 mg/mL. As the introduction of methyl, benthyl, or 4-chlorobenzyl groups in the thiazole ring decreased the antifungal activity of these compounds compared to compound 3a. However, an increase in antifungal activity appeared when coumarin or the benzene ring had an electron-donating group (methyl, methoxyl) or electron-withdrawing group (chloro or bromo). Compound 3f had the most effect against F. moniliforme (inhibitory index: 74%).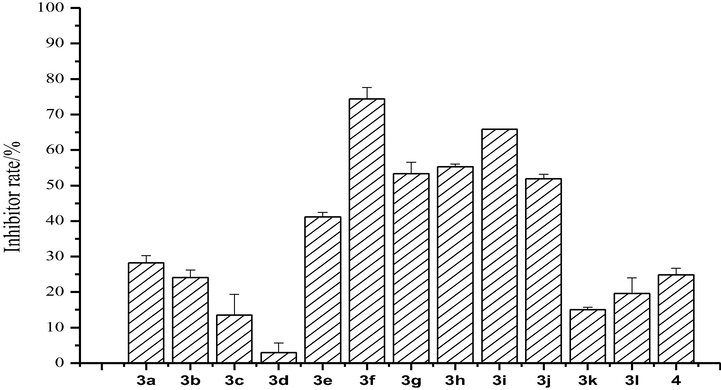
Antifungal activity of the synthesized compounds (3a-3l) against F. moniliforme.
Fig. 4 shows the antifungal activities of the coumarin thiazoles containing a trifluoromethyl group (3a-3 l) against F. graminearum. The inhibitor rate of compound 3a was 22.73% at 0.5 mg/mL. We also demonstrated that the antifungal activities of compounds 3b-3d and 3k-3l were lower than 3a, which means that a substantial group in the 3-position of the thiazole ring or the naphthalene ring in coumarin is unfavorable for the inhibition of F. graminearum. Compound 3g showed the highest inhibitor rate of 89% at 0.5 mg/mL. The reason for this activity may be that the chlorine in coumarin ring decreases the electron cloud density, which increases the combination between the fungicide cell and target molecules.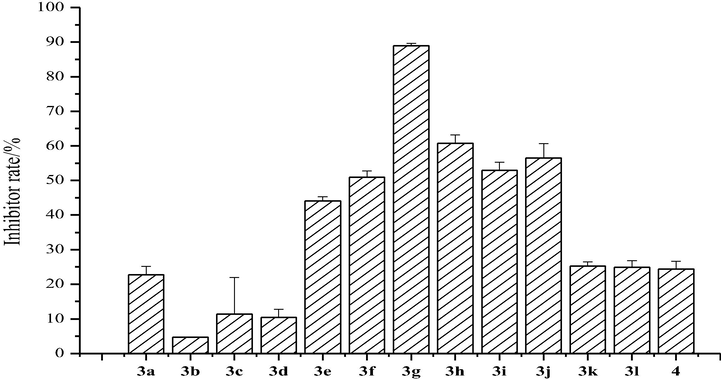
Antifungal activity of the synthesized compounds (3a-3l) against F. graminearum.
For the activities of coumarin thiazoles (3a-3l) against C. lunata (Fig. 5), we found that compound 3g had the highest inhibitor rate of approximately 93.4% at 0.5 mg/mL. Except for compounds 3b and 3l, coumarin thiazoles containing a trifluoromethyl group exhibited higher antifungal activities compared to compound 3a.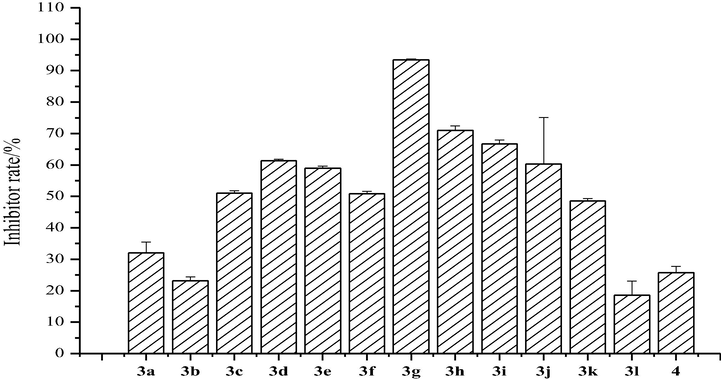
Antifungal activity of the synthesized compounds (3a-3l) against C. lunata.
To investigate the function of trifluoromethyl group, we synthesized the compound 4 without trifluoromethyl group and made a comparison with the corresponding compound 3g. The contrast of the inhibitor rate of compound 3g against F. moniliforme, F. graminearum, C. lunata, respectively (53.4%, 89.0%, 93.4) with that of compound 4 (24.8%, 24.4%, 25.7%) showed that the introduction of trifluoromethyl group increase the antifungal activity of coumarin thiazoles without fluorine. This might be attributed that the trifluoromethyl group improves the lipid solubility of compound 3g (Zhukovskaya et al., 2018).
4 Conclusion
In summary, we efficiently designed and synthesized a novel series of coumarin thiazoles containing a trifluoromethyl group using a one-pot solvent-free protocol. Biological testing data showed that some of the coumarin analogues exhibited good antifungal activity against F. moniliforme, F. graminearum and C. lunata. Compound 3f was identified as the most active against F. moniliforme, and the inhibitor rate was 74%. Compound 3g exhibited the best activity against F. graminearum (inhibitor rate: 89%) and C. lunata (inhibitor rate: 93.4%) and was considered the most promising candidate for further study. The introduction of trifluoromethyl group greatly improved the antifungal activity of coumarin thiazoles. Further structural optimization of coumarin thiazoles containing a trifluoromethyl group is well underway to prepare analogues with improved antifungal activity.
Acknowledgments
This work was supported by Science and Technology Agency of Henan Province (152102110070, 192102110054) and the Guidance Program for Key Scientifific Research Items of Higher Education of Henan Province (20B150008,19B210004).
Declaration of Competing Interest
All authors declare that they have no conflict of interests.
References
- Synthesis and antimicrobial activity of some novel thiazoles, 1,3,4-thiadiazines, 1,3,4-thiadiazoles incorporating coumarin moiety. J. Heterocyclic Chem.. 2015;52:251-259.
- [Google Scholar]
- Heterocyclization of 2-arylidencyclohexan-1,3-dione: synthesis of thiophene, thiazole and isoxazole derivatives with potential antitumor activities. Anti-cancer Agent. Me.. 2019;20:335-345.
- [Google Scholar]
- Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-yl)4-(coumarin-3-yl)thiazoles. Eur. J. Med. Chem.. 2013;62:508-514.
- [Google Scholar]
- Synthesis and biological evaluation of new coumarins bearing 2,4-diaminothiazole-5-carbonyl moiety. Eur. J. Med. Chem.. 2018;155:483-491.
- [Google Scholar]
- Dual evaluation of some novel 2-amino-substituted coumarinylthiazoles as anti-inflammatory–antimicrobial agents and their docking studies with COX-1/COX-2 active sites. J. Enzyme Inhib. Med. Chem.. 2014;29:476-484.
- [Google Scholar]
- New arylpyrazoline-coumarins: synthesis and anti-inflammatory activity. Eur. J. Med. Chem.. 2017;138:170-181.
- [Google Scholar]
- Activity of demethylation inhibitor fungicide metconazole on Chinese Fusarium graminearum species complex and its application in carbendazim-resistance management of fusarium head blight in wheat. Plant Disease. 2019;103:929-937.
- [Google Scholar]
- Antifungal activity, mode of action variability, and subcellular distribution of coumarin-based antifungal azoles. Eur. J. Med. Chem.. 2019;179:779-790.
- [Google Scholar]
- Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. (Weinheim). 2020;353:e1900380
- [Google Scholar]
- Improvement of antagonistic activity of Bacillus megaterium MHT6 against Fusarium moniliforme using He-Ne laser irradiation. Int. J. Agric. Biol.. 2015;17:1141-1148.
- [Google Scholar]
- Synthesis and antimicrobial activity of novel thiazoles with reactive functional groups. Chemistryselect. 2019;4:6965-6970.
- [Google Scholar]
- N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and their analogues: synthesis and multitarget biological activity. Molecules. 2020;25
- [Google Scholar]
- Novel coumarin-thiazolyl ester derivatives as potential DNA gyrase Inhibitors: Design, synthesis, and antibacterial activity. Bioorganic Chem.. 2020;100:103907.
- [Google Scholar]
- Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J.. 2017;25:873-883.
- [Google Scholar]
- New thiazolyl-coumarin hybrids: design, synthesis, characterization, X-ray crystal structure, antibacterial and antiviral evaluation. J. Mol. Struct.. 2018;1166:147-154.
- [Google Scholar]
- A facile one-step multi-component approach toward the synthesis of 3-(2-amino-4-thiazolyl)coumarins by using trimethylsilyl isothiocyanate and their antioxidant and anti-inflammatory activity. Phosph. Sulfur Silicon Related Elements. 2015;190:1393-1397.
- [Google Scholar]
- Synthesis and antiviral activity of a new coumarin derivative against spring viraemia of carp virus. Fish Shellfish Immunol.. 2018;81:57-66.
- [Google Scholar]
- Synthesis of 1-(β-coumarinyl)-1-(β-indolyl)trifluoroethanols through regioselective Friedel-Crafts alkylation of indoles with β-(trifluoroacetyl)coumarins catalyzed by Sc(OTf)3. RSC Adv.. 2020;10:13929-13935.
- [Google Scholar]
- Synthesis and antiviral activity of camphor-based 1,3-thiazolidin-4-one and thiazole derivatives as Orthopoxvirus-reproduction inhibitors. Medchemcomm. 2018;9:1746-1753.
- [Google Scholar]
- Green synthesis and antifungal activities of novel 3-(trifluoromethyl)-4, 5-dihydro-1 H-furo[2,3-c]pyrazole derivatives. J. Heterocyclic Chem.. 2020;57:2904-2910.
- [Google Scholar]
- Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: synthesis, characterization, and antifungal property. Carbohydrate Polym.. 2018;182:180-187.
- [Google Scholar]
- Synthesis of 3-aryl-4-({2-[4-(6-substituted-coumarin-3-yl)-1,3-thiazol-2-yl]hydrazinylidene}methyl/ethyl)-sydnones using silica sulfuric acid and their antidiabetic, DNA cleavage activity. Arabian J. Chem.. 2016;9:S306-S312.
- [Google Scholar]
- Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res.. 2011;21:2217-2222.
- [Google Scholar]
- Synthesis and antibacterial activity of novel chalcone derivatives bearing a coumarin moiety. Chem. Pap.. 2019;73:2493-2500.
- [Google Scholar]
- Design, synthesis and fungicidal activity evaluation of novel pyrimidinamine derivatives containing phenyl-thiazole/oxazole moiety. Bioorg. Med. Chem.. 2019;27:3218-3228.
- [Google Scholar]
- Microwave assisted solvent-free synthesis of 3-(Trifluoroacetyl)coumarins. Chinese J. Org. Chem.. 2015;35:1173-1178.
- [Google Scholar]
- Design, synthesis, antifungal and antibacterial activities of N-phenyl and N-pyridinyl-5-(trifluoromethyl)-pyrazole-4-carboxamide derivatives. J. Heterocyclic Chem.. 2018;55:2261-2269.
- [Google Scholar]
- Synthesis and Antibacterial Activity of N-(4-Substituted phenylthiazol-2-yl)coumarins. Chinese J. Org. Chem.. 2011;31:1930-1933.
- [Google Scholar]
- Synthesis and Pharmacological activity of trifluoromethyl-containing imidazo 1,2-A benzimidazoles. Pharm. Chem. J.. 2018;52:385-391.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.027.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







