Translate this page into:
Electropolishing of pure metallic titanium in a deep eutectic solvent
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This work presents a facile and efficient electropolishing of metallic titanium (Ti) in the relatively eco-friendly electrolyte of deep eutectic solvent (DES) using a mixture of choline chloride-propylene glycol (1 mol ChCl: 2 mol PG). The electropolishing process of metallic titanium was performed under potentiostatic condition (10 V for 40 min) at room temperature. Surface topography and morphology were investigated using scanning electron microscope (SEM) and atomic force microscopy (AFM). The promising electropolishing provides a shiny and mirror-like surface with an average surface roughness (Ra) of 37.92 nm from the parent metallic Ti with a roughness of 455.60 nm. The surface passivation after electropolishing is of the most likely acceptable mechanism for removing microscope roughness. The proposed electropolishing in the present DES electrolyte is a promising strategy for making mirror-like surface (surface resistive against corrosion) of biomedical metallic titanium.
Keywords
Electropolishing
Choline chloride
Propylene glycol
Deep eutectic solvent
Anodisation
Titanium
1 Introduction
Electropolishing is finishing process that atomically removes material from metals and alloys based on anodic dissolution process to make a smooth and bright surface free from grain boundary attack (Karim et al., 2018). Anodic oxidation has been carried out for many metals, such as copper, silver and gold (Karim, 2016; Abbott et al., 2015; Karim et al., 2019). To achieve electropolishing process, a viscous layer has to be formed at electrolyte/electrode interfacial region. This is to control mass transport of ions in which the surface roughness attenuation can be reached. It is well-known that after electropolishing, the surface resists corrosion relatively for reasonable long period of time (Han and Fang, 2019; Abbott et al., 2006a, 2006b).
Titanium as one of precious metals has wide applications, such as in aerospace and medicine (Gurappa, 2002; Ryhänen et al., 1997). It has been polished electrochemically in perchloric acid-based electrolytes, such as perchloric acid/acetic acid and perchloric acid/methanol/ethylene glycol systems (Mathieu and Landolt, 1978; Peighambardoust and Nasirpouri, 2014). It has also been electropolished in perchloric acid-free electrolytes, for instance methanol/sulfuric acid and ethylene glycol/NaCl (Piotrowski et al., 1998; Kim et al., 2015).
Ionic liquids are defined as salts with relatively high conductivity compared to organic electrolyte media with low melting point below the boiling point of water (Smith et al., 2014; Endres et al., 2017). Over last two decades, these electrolytes have been used as a proper medium for electrochemical reactions and show wide potential windows in electrochemical course (Abbott et al., 2013; Abbott et al., 2017) Deep eutectic solvents (DESs) are sub-class of ionic liquid with more environmental friendly than ionic liquids. From choline chloride that is a quaternary ammonium salt containing, one can make a range of deep eutectic solvents using metal salts and hydrogen bond donors (Smith et al., 2014).
In this work, electropolishing of metallic titanium in a mixture of choline chloride-propylene glycol (1 ChCl: 2 PG) was successfully conducted. This finding is of significant importance for industrial and biomedical applications in which the smooth surface resists corrosion. Under electrochemical conditions of relatively low potential (10 V) and for 40 min, and mol ratio of 1 ChCl: 2 PG, a mirror-like finished surface was obviously obtained.
2 Experimental
2.1 Chemicals
The Ti sheet was obtained from Alfa Aesar (Alfa Aesar 99%, USA). Prior to use, the Ti sheet was properly cleaned in mixture of dilute sulfuric acid and hydrochloric acid. Afterwards, it was inserted into acetone with sonication in order to remove impurities. The propylene glycol (PG, 99%, Sigma Aldrich, USA) and choline chloride (ChCl, 99%, Sigma Aldrich) were purchased from Sigma Aldrich (Sigma Aldrich, USA) and used as received.
2.2 Instruments and measurements
The current density-voltage (I-V) and current density-time (I-t) plots were drawn using AC/DC Laboratory Power Supply DC: 0–30 V and digital multi-meters (Widewing AN8008 True-RMS). For morphological analysis, scanning electron microscopy (SEM) was used ex situ to investigate the surface morphology (Philips XL30 ESEM). The atomic force microscopy (AFM) image acquisitions were recorded ex situ using a Digital Instruments Nanoscope 4 run at contact mode operation. The AFM images of titanium recorded in resonant mode at a frequency (300 Hz) at a scan rate of 0.5 Hz.
2.3 Preparation of deep eutectic solvent
The preparation of deep eutectic solvent was performed by mixing choline chloride (ChCl) and propylene glycol (PG) in a 1:2 mol ratio. The mixture was heated at 60 °C with continuous stirring till a homogeneous clear electrolyte was gained.
2.4 Electrochemical polishing
For the electropolishing process, 1 cm2 Ti disk (thickness of 2 mm) and cylindrical platinum electrode were used as working and counter electrodes, respectively in 10 ml of the electrolyte ChCl: PG in 1:2 mol ratio at a constant potential of 10 V for 40 min at room temperature. The distance of 1 cm was kept between the working and the counter electrodes.
3 Results and discussion
3.1 Electrochemical study
The electrochemical response of pure metallic titanium in ChCl: PG in 1:2 mol ratio at 20 ○C is presented in Fig. 1a. It is seen that there is no noticeable dissolution electrochemically in the potential range of 0.1–5 V as shown in region A. Within 5–9 V presented in region B, there is a random electrochemical dissolution leading to pitting other than desired polishing. From 9 to 12 V that exhibited in region C, the effective electropolishing process occurs whereas beyond 12 V shown in region D, huge electrolyte decomposition takes place. It is worth-mentioning that at the constant current plateau (ca. 9–12 V), mass transport is responsible for achieving electropolishing successfully. This electrochemical dissolution of Ti is believed to be due to complex formation with Cl− ion as shown in the following equation:
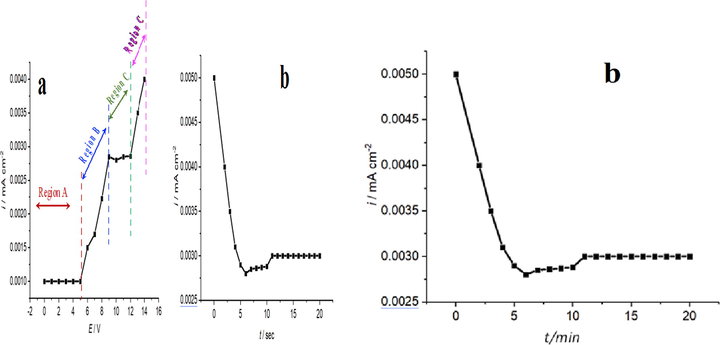
Shows electrochemical studies of metallic Ti in ChCl: PG in 1:2 mol ratio; polarisation curve determined (a) and i-t curve measured at constant potential of 10 V for 20 min. (b).
This electrochemical reaction would be accelerated in the presence of the Cl− ions (choline chloride) from the bulk electrolyte region to interfacial region (Han and Fang, 2019). The constant current plateau (i.e. steady state) is the desired region where electropolishing takes place effectively, and the steady state faradaic current is the mass transport controlled. The electrolyte decomposition might involve H2 evolution where the propylene could be the main source in addition to the presence of trace water in the mixture (Haerens et al., 2009; Abbott et al., 2006a, 2006b).
Fig. 1b shows the I-t plot of Ti in ChCl: PG in 1:2 mol ratio at 20 ○C. It is seen that after 6 min of potential holding at 10 V, a steady state reaches. This indicates the real electropolishing begins beyond this period and continues until the end of time scale of the experiment. The potential holding in electrochemical course is helpful to accumulate desired ion with enough concentration at the interface region where the electropolishing occurs.
The graphical representation of electrochemical polishing under mass transport is presented in Fig. 2 Based on the viscous film theory, the film is relatively thick in the valley positions (Fig. 2, shown in b arrow) and the metal dissolution is slow due to high electric resistance and low potential distribution (Han and Fang, 2019). On the contrary, at the protruding positions (shown in a arrow) the viscous film is thin and the metallic dissolution is fast owing to low electric resistance and high potential distribution.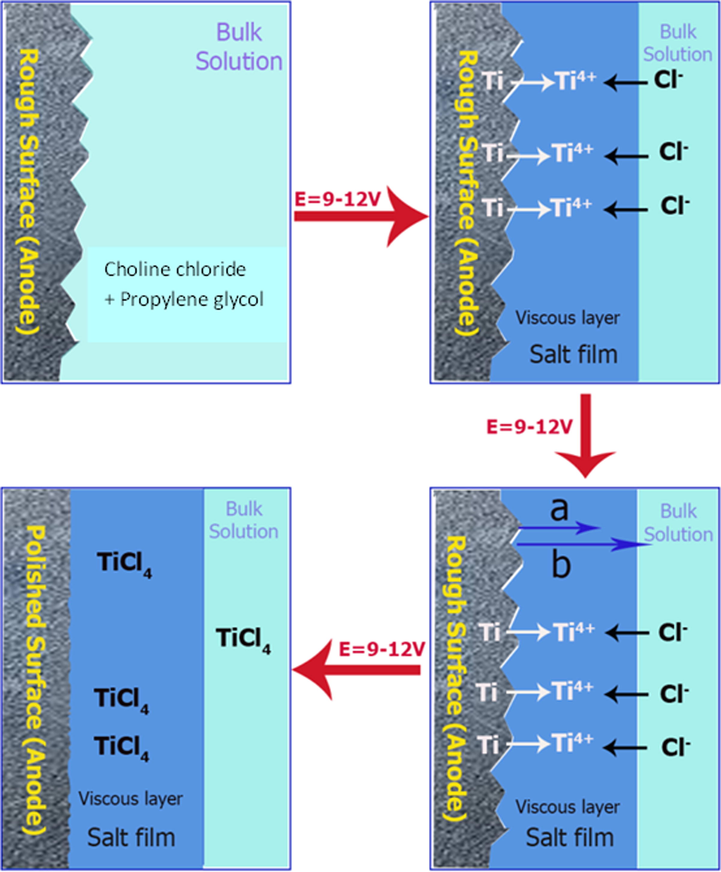
Typical schematic diagram shows the mechanism of electropolishing.
3.2 Surface morphology
3.2.1 Scanning electron microscope (SEM)
The electropolishing course of Ti sheet was conducted in propylene-choline chloride electrolyte by holding potential at 10 V for 40 min as shown in a micrograph in Fig. 3. The optimum potential range was obtained which is from 9 to 12 V for 40 min. It is obviously seen that the features of polished and unpolished at the boundary are definitely shown in the SEM micrographs of the Ti surface. From the SEM image, it is seen that there is a considerable change of Ti surface from large grain surface to a large extent to a surface that free from grain crystal surface.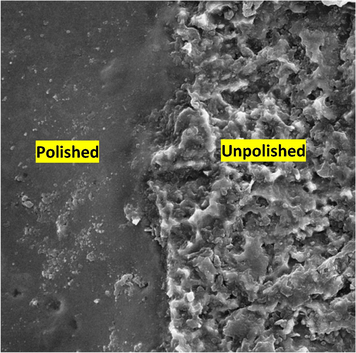
SEM image at the boundary between the unpolished and polished metallic Ti sample in 1 ChCl: 2 PG at 10 V for 40 min at 20 °C.
In fact, before the effective electropolishing potential (ca 9 V), a dull surface finished was gained on the Ti surface.
3.2.2 Atomic force microscopy (AFM)
Fig. 4 exhibits Ti sheet after electropolishing in 1 ChCl: 2 PG mol ratio mixture at 20 °C. From the image it is clearly seen that the roughness is considerably reduced. The interesting observation is this large reduction in surface roughness from parent metallic Ti sheet. The polished surface roughness obtained for Ti is quite close to this obtained for Ni and Co (Karim et al., 2018). The surface roughness of the Ti sheet prior to electrochemical polishing is about 455.60 nm where reduced to an average roughness of 37.92 nm (over five measurements).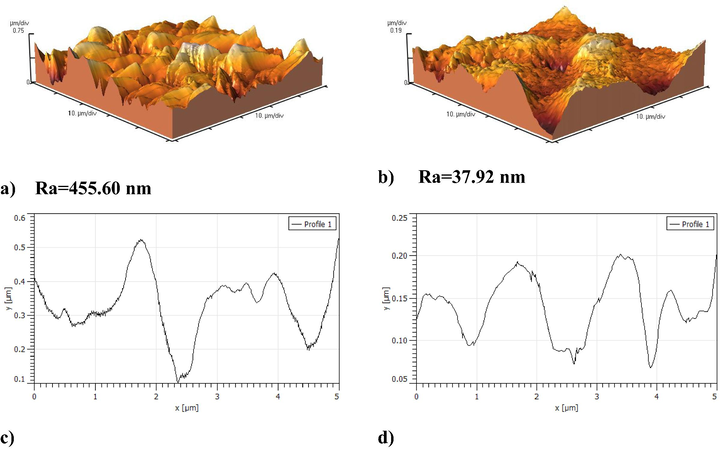
AFM images of a sample of Ti sheet after dissolution in 1 ChCl: 2 PG at 10 V for 40 min at 20 °C, a) Parent Ti sheet, b) Ti sheet after electropolishing, c and d) single line traces alongside of Ti recorded before and after electropolishing, respectively in resonant mode at a frequency of ca. 300 Hz (recorded in air at a scan rate of 0.5 Hz, 256 lines).
The single line traces alongside of metallic titanium before and after electropolishing is presented in Fig. 4c and d. The surface is relatively very rough with grain size of up to 600 nm where converted to 250 nm in the polished one. The cross section of Ti corresponding to a single line at X-axis of 5000 nm in both cases was scanned. Over the Y-axis, in the polished one, there is a reduction in the grain sizes up to 200 nm.
In the Fig. 5, a photograph exhibits a clear mirror-like surface that obtained at a constant potential of 10 V at for 40 min at 20 ○C.
Shows an optical photograph of a sample of Ti both unpolished and polished regions in 1 ChCl: 2 PG.
4 Conclusions
Summarily and descriptively, the following points have been concluded:
-
The electropolishing of metallic titanium produces a relatively high-quality bright surface with uniform surface morphology.
-
It seems that propylene glycol as efficient as ethylene glycol to interact with choline chloride in 1:2 mol ratio to form a deep eutectic solvent.
-
The optimum potential range has been confirmed that effective electropolishing occurs.
-
It is also emphasised that the eutectic mixture provides sufficient viscous medium where Ti can be electropolished by facilitating mass transport throughout the interfacial region.
-
The electropolishing of Ti in this electrolyte results in leveling and disappearance of surface defects.
-
The anodic potential of the process is relatively low (10 V for 40 min) which is necessary to cause anodic film breakdown that prevents the metal from dissolution.
Acknowledgements
I would like to acknowledge the College of Education and College of science in the University of Sulaimani for giving this opportunity to conduct this research.
Declaration of Competing Interest
I have no conflicts of interest to disclose.
References
- Electropolishing of stainless steels in a choline chloride based ionic liquid: an electrochemical study with surface characterisation using SEM and atomic force microscopy. Phys. Chem. Chem. Phys.. 2006;8:4214-4221.
- [CrossRef] [Google Scholar]
- Voltammetric and impednace studies of the electropolishing of type 316 stainless steel in a choline chloride based ionic liquid. Electrochem. Acta.. 2006;51:4420-4425.
- [CrossRef] [Google Scholar]
- Abbott A.P., Karim W.O., Ryder K.S., 2017. Technical aspects, Electrodeposition from Ionic Liquids. P 341.
- Electroplating using ionic liquids. Annu. Rev. Mater. Res.. 2013;43:335-358.
- [CrossRef] [Google Scholar]
- Anodic dissolution of metals in ionic liquids. Prog. Nat. Sci.. 2015;25:595-602.
- [CrossRef] [Google Scholar]
- Electrodeposition from Ionic Liquids. John Wiley & Sons; 2017.
- Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact.. 2002;49:73-79. http://doi.org/10.1016%2FS1044-5803(02)00320-0
- [Google Scholar]
- Electrochemical decomposition of choline chloride based ionic liquid analogues. Green Chem.. 2009;11:1357-1365.
- [CrossRef] [Google Scholar]
- Fundamental aspects and recent developments in electropolishing. Int. J. Mach. Tools Manuf.. 2019;139:1-23.
- [CrossRef] [Google Scholar]
- Anodic Dissolution of Metals in Ionic Liquids. University of Leicester; 2016. PhD Thesis
- Electropolishing of nickel and cobalt in deep eutectic solvents. Trans. Inst. Met. Finish.. 2018;96:200-205.
- [CrossRef] [Google Scholar]
- The anodic behaviour of bulk copper in ethaline and 1-butyl-3-methylimidazolium chloride. Appl. Sci.. 2019;9:4401-4415.
- [CrossRef] [Google Scholar]
- The effect of added ethanol in ethylene glycol-NaCl electrolyte on titanium electropolishing. Corros. Sci.. 2015;98:1-25.
- [CrossRef] [Google Scholar]
- Mathieu, J.B., Landolt, D., 1978. Electropolishing of titanium in perchloric acid-acetic acid solution: II. Polarization behavior and stoichiometry. J. Electrochem. Soc. 125, 1044-1049. https://doi.org%2F10.1149%2F1.2131618.
- Electrochemical behaviour of pure titanium in perchloric acid-methanol-ethylene glycol mixed solution. Trans. Inst. Met. Finish.. 2014;92:132-139.
- [CrossRef] [Google Scholar]
- The mechanism of electropolishing of titanium in methanol-sulfuric acid electrolytes. J. Electrochem. Soc.. 1998;145:2362-2369.
- [Google Scholar]
- Biocompatability of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res.. 1997;35:451-457.
- [CrossRef] [Google Scholar]
- Deep eutectic solvents (DESs) and their applications. Chem. Rev.. 2014;114:11060-11082.
- [CrossRef] [Google Scholar]







