Translate this page into:
In silico exploration of anti-Alzheimer's compounds present in methanolic extract of Neolamarckia cadamba bark using GC–MS/MS
⁎Corresponding author. ksrao@igntu.ac.in (Srinivasa Rao Kareti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer’s disease (AD) can be treated by the inhibition of Beta Amyloid protein (Aβ) and inhibition of Acetylcholinesterase (ACHE). Anti-Alzheimer’s potential phytoconstituents from Neolamarckia cadamba methanolic bark extracts were identified through GC–MS/MS analysis and in silico molecular docking analysis. Powdered bark sample was subjected to extract by soxhlet extractor with n-hexane, chloroform and methanol solvents respectively. The methanolic extract was taken for GC–MS/MS analysis, the observed chromatogram was revealed the presence of 61 constituents in the methanolic extract, 59 new phytoconstituents were identified which were not reported earlier as constituents any part of N. cadamba. GC–MS/MS detected phytoconstituents were analysed through the docking analysis by iGEMDOCK software against Aβ (PDB ID: 2LMN) and ACHE (PDB ID: 3LII) and compared with standard known inhibitors of galantamine and curcumin. Docking analysis binding energy was determined and verified by Discovery studio visulaizer. Both inhibition assay top 5 best dock energy compounds were analysed through the in silico modeling through admetSAR web portal for parameters of intestinal absorption, blood brain barrier permeation, carcinogencity, and acute oral toxicity were determined. From that heptadecanoic acid, 16-methyl-, methyl ester; beta-sitosterol acetate and octadecanoic acid, 2-hydroxy-, methyl ester inhibitors were identified. Further the top lead successful compound of each target molecular interactions were detected by LigPlot analysis. From this research these three compounds are best to treat AD than standard. Isolation of individual compounds would, however, help to find new compounds for other diseases and lead molecules for AD were identified.
Keywords
Anti-Alzheimer’s
Neolamarckia cadamba
β amyloid inhibition
Acetylcholinesterase inhibition
Gas Chromatography-Mass Spectrometry (GC–MS/MS)
1 Introduction
Neolamarckia cadamba (N. cadamba) species belongs to Rubiaceae family which is known as kadamba. The synonyms used for this plant are Anthocephalus cadamba, Anthocephalus indicus, Sarcocephalus cadamba (Roxb). It is a tropical tree species that is native to South Asia and Southeast Asia, which is widely distributed in moist deciduous evergreen forests and widely distributed throughout the greater part of India (Umachigi et al., 2007). It is a large tree with broad spreading branches and grows rapidly in the first 6–8 years. The tree usually reaches a height of 45 m with a stem diameter of 100–160 cm (Krisnawati et al., 2011). It has scented orange flowers present in dense globe-shaped clusters, which are used in the preparation of perfumes. It is an ornamental plant that is also used for timber and paper-making (Dwevedi et al., 2015). It is used widely in Indian traditional formulations i.e in ayurveda formulations stomatitis, obesity, Charaka Samhita mentions the use of N. cadamba bark for the strengthening the body immunity, as an analgesic and for the treatment of fever and urinary troubles. Shukla Yajurveda describes the use of pollens of N. cadamba for strengthening the body and mind, Brahmavaivarta Purana mentions that beneficial for teeth (Pandey and Negi, 2016), The Ayurvedic Pharmacopoeia of India indicates the use of d’ried stem bark in disorders of female genital tract and bleeding disorders (Khare, 2013), and it has been a remedy in the treatment of eye infection, skin diseases, dyspepsia as well as to cure the gum related troubles, stomatitis, cough, fever, anaemia, blood disorders, stomach pain (Umachigi et al., 2007). Stem barks are having febrifugal, antidiuretic, anthelmintic, hypoglycaemic effects. Fruits are used for their cooling effect, anticatarrhal, blood purifier, and analgesic purposes. Flowers and roots are having abortifacient effects. Leaves are having astringent, gargling in stomatitis and treat aphthae. The crude extracts from N. cadamba have been shown to possess biological activities viz., anti-inflammatory (Chandrashekar et al., 2010), antihepatotoxic activities (Kapil et al., 1995), anti-Inflammatory, analgesic and antipyretic (Verma et al., 2019), hypoglycemic activity, oxidative stress (Alam et al., 2011), antimicrobial, anthelmintic activities (Usman et al., 2012), sedative and antiepileptic effects (Nagakannan et al., 2011).
The bark extracts of N. cadamba revealed the presence of various secondary metabolites like glycosides, alkaloids, tannins, phenolic; steroids; flavonoids (Usman et al., 2012)., quinoline alkaloids (Handa et al., 1984) and triterpene glycosides (Sahu et al., 2000). From the bark extracts hexadecanoic acid, methyl ester; heptadecanoic acid, ethyl ester; stearic acid, methyl ester; octadecanoic acid, ethyl ester; docosanoic acid, methyl ester; 1,2-benzenedicarboxylic acid; tricosanoic acid, methyl ester; pentacosanoic acid, methyl ester; tetratetracontane, diisooctyl ester; heneicosane; octadecanoic acid, methyl ester; eicosane; benzaldehyde; benzyl alcohol; pentanoic acid; 4-oxo-, phenylmethyl ester; benzyl ether; tetradecanoic acid; n-hexadecanoic acid; progesterone; tetratetracontane; dodecanoic acid; myristic acid; 2-cyclohexen-1-one; 4-hydroxy-3,5,5-trimethyl-4-(3-oxo-1-butenyl); pentadecanoic acid; n-hexadecanoic acid; hexadecanamide and octadecanamide (Ho et al., 2014), 3b-isodihydrocadambine; aminocadambines A and B; neolamarckines A and B; cadamine, cadambine; isocadambine (Raja et al., 2013); isodihydrocadambin and isodihydrocadambine (Brown et al., 1974). Traditional medicinal plants were the first choice for addressing problems such as infertility, impotence, diabetes, obesity, psychosomatic troubles, epilepsy and several other diseases (Said et al., 2002).
Alzheimer's disease (AD) is a dynamic neurodegenerative issue with age-related symptoms, including cognitive impairment, psychosis, over activity, aggressive behaviour, and depression (Hope et al., 1999). Late-onset or sporadic AD is presently cured by using AChE inhibitors (Mcgleenon et al., 1999) and Aβ inhibitors (Sharma et al., 2017), both of which have serious side effects (Casey et al., 2010). WHO reported that 36 millions of people were living with dementia in 2010, and that this number is almost expected to be double every 20 years, reaching 66 million by 2030 and 115 million by 2050 (Prince, 2009). AD brain tissue studies revealed that the enzymes that produce and utilize acetylcholine are considerably diminishing to a significant level in AD patients (Hope et al., 1997). The brains of AD patients typically exhibit significant neurodegeneration associated with atrophy of the cerebral hemispheres, cortex, white matter and ventricular enlargement, coincident with extracellular plaques consisting mostly of amyloid-β peptides and intracellular neurofibrillary tangles associated with hyper phosphorylated tau peptides (Davies and Maloney, 1976; Hubbard et al., 1990).
Acetylcholinesterase inhibitors such as donepezil, rivastigmine and galantamine inhibit the action of acetylcholinesterase (AChE) in the synapse by preventing its degradation. AChE inhibitors are generally well-tolerated, but common side effects include nausea, vomiting, loss of appetite, diarrhoea and bradycardia. Nevertheless, they show modest clinical benefits in the earlier stages of AD (Lanctot et al., 2003). N-methyl-D-aspartate receptor antagonists manage levels of the neurotransmitter glutamate. The approval of memantine in Europe in May 2002 and in US October 2003 has transformed the treatment of moderate to severe AD. Moreover, Daiichi-Sankyo is developing memantine for the Japanese market, where it is currently in phase 3 clinical developments for the treatment of severe AD (Veerman et al., 2016). Memantine is also having side effects of headache, insomnia, constipation, fatigue, dizziness, auditory hallucinations and mild allergic symptoms (Iorio et al., 2017; Lieberman et al., 2009). Decreasing amyloid β (Aβ) levels in the brain will reduce the risk for AD (Hartman, 2009). BACE-1 (β-site APP cleaving enzyme) is considered a primary target for preventing the accumulation of Aβ plaques (Thompson et al.,2005; Vassar and Kandalepas, 2011; Ghosh and Sandra, 2008). Many peptidomimetics and heterocyclic compounds have been assessed as BACE-1 inhibitors (Mancini et al., 2011; Varghese, 2006).
Therefore, there is a high demand to develop new anti-AD drugs. In recent years the use of medicinal plants in the management and treatment of several diseases has gained considerable importance. Plants are considered as one of the main sources of biologically important active constituents. It is an estimate of WHO, Geneva that 85–90% of the world population relies on traditional medicine to meet their primary health care demands (Okigbo et al., 2009). It is a known fact that plants are having secondary metabolites that are responsible for the therapeutic activity of that particular plant material (Ji, 2001).
A very few reports are found on the chemical constituents of N. cadamba for different parts of plant. The main objective of the present study was to analyse secondary metabolites present in methanolic bark extract of N. cadamba using GC–MS/MS. We have compared previous reported chemical constituents data with the GC–MS/MS data of N. cadamba methanolic bark extract and also examine the binding capacity of these phytoconstituents with Aβ and ACHE to explore their AD inhibitory activity. Moreover, our investigation may facilitate further development of more potent anti-Alzheimer’s drugs.
2 Materials and methods
2.1 Chemicals and instrumentation
Solvents for the extraction were purchased from Loba Chemie Pvt. Ltd., Mumbai, India, and are used without further purification. All the solvents were of analytical grade. Chromatographic analyses were performed on a Shimadzu GCMS-QP2010 Plus gas chromatograph–mass spectrometer system (Shimadzu, Japan) with a programmed temperature vaporizer injector (PTV) and an AOC-20i auto sampler. A personal computer equipped with the GC–MS system was used to process the MS data.
2.2 Collection and authentication of plant material
N. cadamba fresh bark was collected during April month of 2018 at Amarkantak hills, Anuppur district, Madhya Pradesh, India. The collected bark was washed thoroughly by running tap water to remove sand particles and other adhered debris and finally washed with sterile distilled water. The plant material is pulverised to powder and then stored it in an airtight container. The plant material was authenticated by taxonomist and herbarium specimen voucher (DOB/Herb/Rub-01) has been deposited at Indira Gandhi National Tribal University, Amarkantak, India.
2.3 Soxhlet extraction
Powdered sample (100 g/250 ml) was extracted with soxhlet extractor by three different solvents namely n-hexane, chloroform, and methanol respectively at 50–75 °C for 12 h of extraction in order to extract nonpolar to polar compounds. The collected extracts were dried under water bath below 60 °C and stored it in a refrigerator.
2.4 GC–MS/MS analysis
Methanolic extract of N. cadamba was taken for GC–MS/MS analysis, GC–MS/MS analysis was performed on (Shimadzu) GCMS-QP2010 Plus instrument, injection temperature was maintained at 280 °C and the injection volume of the analytical solution was 2.0 µL, the split ratio was 1:3; the oven temperature program was set initially at 50 °C (held for 1 min) and increasing it at a rate of 7.5 °C per min up to 300 °C for 10 min. High pure helium was used as inert gas, the ion source temperature is maintained at 250 °C and the interface temperature was set 300 °C. The mass spectrometer (MS) was operated in an acquisition (ACQ) mode for quantitative analysis. Mass spectral data were obtained from the full scan range of m/z 50–1000 in order to obtain the fragmentation spectra of the target analytes.
2.5 Experimental
2.5.1 Hardware specification
Molecular modeling studies were carried out on an Intel (R) Core (TM) running windows 10.
2.5.2 Software specifications
Receptor protein structures were downloaded from the https://www.rcsb.org. Screening of the receptor structures was done by PyRx 0.8 software, which was downloaded from https://pyrx.software.informer.com. GC–MS/MS identified compound structures were drawn by using Chemdraw 19.1 trail license version obtained from PerkinElmer, United States. iGEMDOCK V2.1 program was used for molecular docking studies, which was downloaded from http://gemdock.life.nctu.edu.tw. Discovery studio visualizer V20 was downloaded from https://www.3dsbiovia.com for docking verification. Online SMILES translation was carried out using https://cactus.nci.nih.gov/. ADMET studies were determined by admetSAR using www.lmmd.ecust.edu.cn web portal and LigPlot analysis was performed by LigPlot + V 2.1 licensed version obtained from European Bioinformatics Institute, United Kingdom.
2.5.3 Preparation of receptors
The receptors of Aβ (PDB ID: 2LMN) and ACHE (PDB ID: 3LII) structures were retrieved from the protein data bank (PDB) database. All non-protein molecules and any other alternative atom locations were removed from 2LMN and 3LII; these protein receptor structures were screened by using PyRx 0.8 software (Dallakyan and Arthur, 2015).
2.5.4 Preparation of ligand
The ligand preparation process consisted of various steps that perform conversions, corrections and variations of the structures, elimination and lead optimization. The ligands structure were drawn using the ChemDraw and then it was converted into 3D mol format (Mendelsohn, 2004). The conversion of the SMILES structures from 3D mol format by using cactus smile converter tool (Suri and Naik, 2012), the addition of hydrogen atoms, removal of heteroatoms, neutralization of charged groups, generation of ionization states and tautomers, filtration, alternative chiralities, optimization of geometries, low-energy ring conformers and finally conversion and saving of output files were performed during ligand preparation (Usha et al. 2013). Docking studies allows implicitly screening a compound database and prediction of the strongest binders based on their scoring functions.
2.5.5 Molecular docking studies
Molecular Docking is the process by which two molecules fit together in 3D space; it is a key tool in structural biology and computer-aided drug design (Palleti et al., 2011; Ladokun et al., 2018). In order to get an accurate docking, stable standard dock was used as a default setting. In iGEMDOCK, the docking run parameters were set as population size (N = 200), generations (70), number of solutions (2). The best pose ligands were selected based on their best conformation that allows the lowest free energy of binding (Kandeel and Kitade, 2013).
For protein–ligand docking studies, the selected iGEMDOCK provides an interactive interface for the preparation of the binding site and ligand docking status and monitoring the progress. For most docking study tools, users usually need to prepare the structure of the binding site and ligand. The selected software provides a straightforward method to derive the binding site from the bounded ligand and it also able to automatically consider the effects of hydrogen atoms on the binding sites. After the screening process, it utilizes the post-screening analysis module to infer pharmacological interactions and cluster screened compounds based on protein–ligand complexes and compound structures. The atomic composition is useful for measuring compound similarity. It provides an interactive interface for visualizing compound similarity with a hierarchical tree by Java tree view. Finally, iGEMDOCK ranks and visualizes the screened compounds by combining the pharmacological interactions and the energy-based scoring function (Hsu et al., 2011). The results obtained from iGEMDOCK were analysed to study the binding energy and the interactions of the docked structure.
2.5.6 In silico ADME-Toxicity
To determine which lead compounds possess the drug-likeness characteristics and should thus become a focus of subsequent analyses, we performed in silico ADMET assays for the compounds with the most promising structural backbones (Zhang et al., 2017). ADMET studies have shown (absorption, distribution, metabolism, excertion and toxicity) properties that are necessary and helpful in developing new natural compounds with enhanced pharmacokinetic and pharmacodynamic properties. ADMET prediction includes intestinal absorption, blood–brain barrier (BBB) penetration, carcinogenicity and acute oral toxicity of top five phytoconstituents were estimated to test their the drug-likeness using admetSAR server (Alhazmi, 2015; Cheng et al., 2012).
2.5.7 LigPlot analysis
The LigPlot program automatically generates schematic 2-D representations of protein–ligand complexes from standard PDB file input. The output is a colour or black-and-white file giving intermolecular interactions like hydrogen bonds and hydrophobic interactions and also it provides their strengths. This program is completely general for any ligand and can be also be used to show other types of interaction in proteins and nucleic acids (Wallace et al., 1995). The LigPlot analyses were introduced to understand the in-depth interaction pattern between the docked ligands and the active site residues (Bharatham et al., 2008).
3 Results
3.1 Compound identification by GC–MS/MS
The characteristic mass fragments used for quantitative and qualitative analysis and the retention times revealed the presence of sixty one phytoconstituents from the bark of N. cadamba as shown in supplementary Table 1. Only 2 constituents were reported earlier namely hexadecanoic acid and hexadecanoic acid, methyl ester remaining 59 new phytoconstituents were detected. The compounds prediction and their identification were done on the basis of NIST14.lib and WILEY8.Lib. GC–MS chromatogram of methanolic extract of N. cadamba is shown in Fig. 1 and the majority of phytoconstituents fragmentation pattern and their MS spectral data are shown in supplementary Fig. S1.
Dock score Rank
Compound No
Top five compounds with 2LMN
Intestinal absorption
BBB permeation
Caricino genicity
Acute oral Toxicity (Kg/mol)
1.
51
Eicosanoic acid, methyl ester
+
+
+
2.828
2.
58
9-Octadecenamide
+
+
+
2.524
3.
46
9,12-Octadecadienoic acid, methyl ester, (E,E)
+
+
+
0.5714
4.
49
Heptadecanoic acid, 16-methyl-, methyl ester
+
+
–
2.701
5.
40
Hexadecanoic acid, methyl ester
+
+
+
0.5714
Top five compounds with 3LII
1.
60
Beta-sitosterol acetate
+
+
–
3.651
2.
58
9-Octadecenamide
+
+
+
2.524
3.
51
Eicosanoic acid, methyl ester
+
+
+
2.828
4.
57
Octadecanoic acid, 2-hydroxy-, methyl ester
+
+
–
2.799
5.
46
9,12-Octadecadienoic acid, methyl ester, (E,E)
+
+
+
0.5714
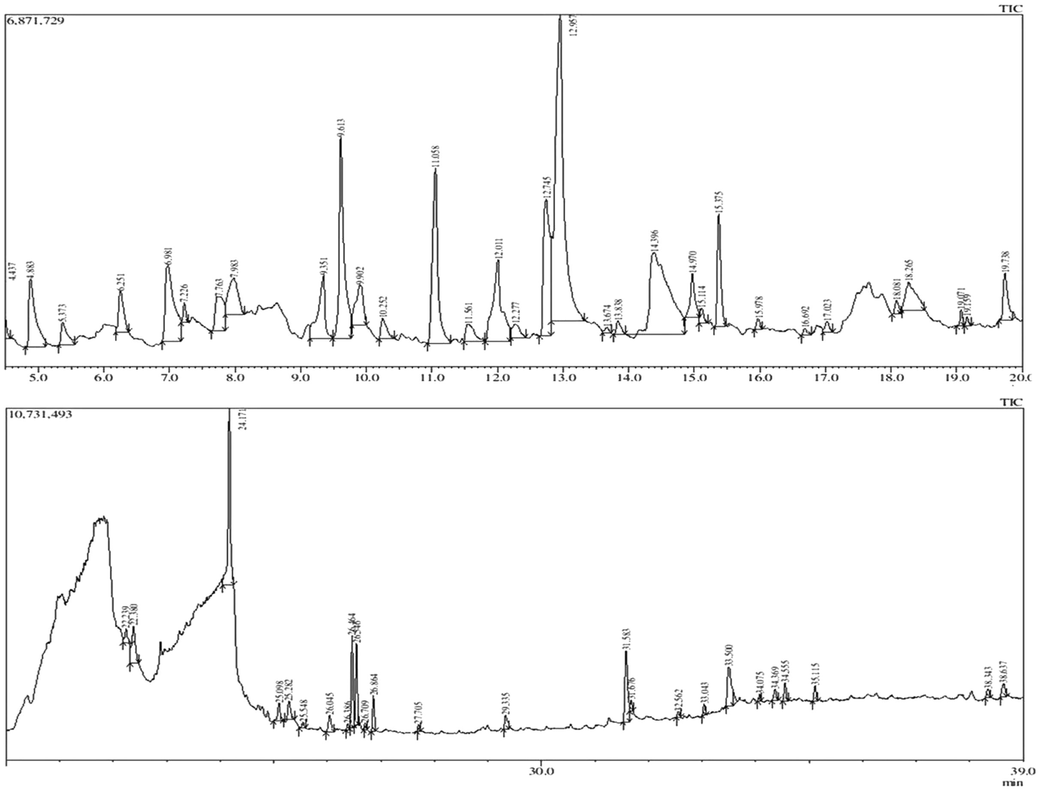
GC–MS/MS Chromatogram of methanolic extract of N. cadamba bark.
3.2 Ligand preparation
Supplementary Fig. S2 shows the prepared ligands present in methanolic extract of N. cadamba using GC–MS/MS.
3.3 Molecular docking study
Best pose dock score compounds were predicted for the identified phytoconstituents for their interaction with AD targets of Aβ protein (PDB ID: 2LMN) and ACHE (PDB ID: 3LII) Fig. 2. Methanolic extract of N. cadamba phytoconstituents ligands were screened against the selected two AD receptor targets and their results are shown in supplementary Fig. S3. Supplementary Table 1 shows the binding energy of ligand-receptor interactions of all phytoconstituents. The phytoconstituents were ranked based on their post docking analysis and hierarchical clustering of compounds. A decrease in intermolecular energy for all the selected phytoconstituents with a simultaneous reduction in their binding energy is observed. This result further emphasizes the AD inhibitory activity of all the selected phytoconstituents.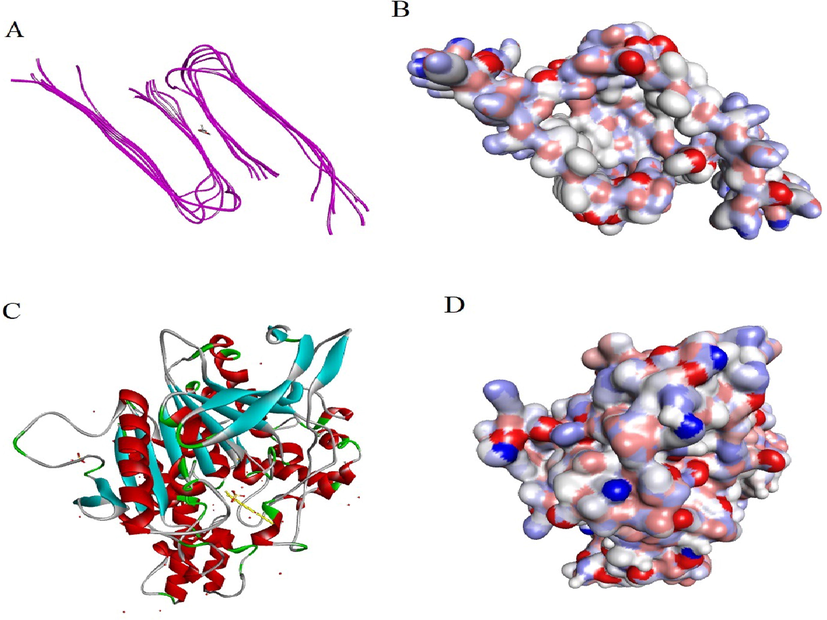
Molecular docking view: A. Chain 2LMN interacting with heptadecanoic acid, 16-methyl-, methyl ester. B. Surface of 2LMN protein with heptadecanoic acid, 16-methyl-, methyl ester. C. Chain 3LII interacting with beta-sitosterol acetate. D. Surface of 3LII protein with beta-sitosterol acetate.
3.3.1 Top dock score phytoconstituents interaction with 2LMN
Nine phytoconstituents were exhibited highest docking score and lowest binding energy compared to standard curcumin (−110.2 kcal/mol). They are namely eicosanoic acid, methyl ester (−141.4); 9-octadecenamide (−137.1); 9,12-octadecadienoic acid, methyl ester (E,E) (−131.4); heptadecanoic acid, 16-methyl-, methyl ester (−123.5); hexadecanoic acid, methyl ester (−120.2); trichloroacetic acid, pentadecyl ester (−118.6); hexadecanoic acid (−112.4); hexadecanal (−111.1); octadecanoic acid, 2-hydroxy-, methyl ester (−110.4).
3.3.2 Top dock score phytoconstituents interaction with 3LII
Twenty one phytoconstituents were exhibited highest docking score and lowest binding energy compared to standard galantamine (−74.5). They are namely beta-sitosterol acetate (−93.9); 9-octadecenamide (−91.3); eicosanoic acid, methyl ester (−90.3); octadecanoic acid, 2-hydroxy-, methyl ester (−88.9); 9,12-octadecadienoic acid, methyl ester, (E,E) (−87); heptadecanoic acid, 16-methyl-, methyl ester (−86.1); trichloroacetic acid, pentadecyl ester (−85.6); hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl (−85.1); (9E,12E)-9,12-octadecadienoyl chloride (−83.4); 9,12-octadecadienoic acid (z,z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester (−82.7); 1-hexadecanol, 2-methyl-(−82.1); hexadecanoic acid, methyl ester (−81.2); hexadecanoic acid, (3-bromoprop-2-ynyl) ester (−80.9); hexadecanoic acid, 2-hydroxy-, methyl ester (−80.6); vitamin e (−78.9); phytol (−78.3); hexadecanoic acid (−77.3); squalene (−77.1); pentadec-7-ene, 7-bromomethyl (−74.9); diethyl phthalate (−74.6) and hexadecanal (−74.6).
3.3.3 Prediction of binding sites
The binding sites present in 2LMN and 3LII protein receptors were predicted with the least binding energy indicates an active site where targeted ligand molecule can bind or interactions are shown in Fig. 3A and B. The binding interaction between ligand-receptor active sites, which were predicted by Discovery studio visualizer software.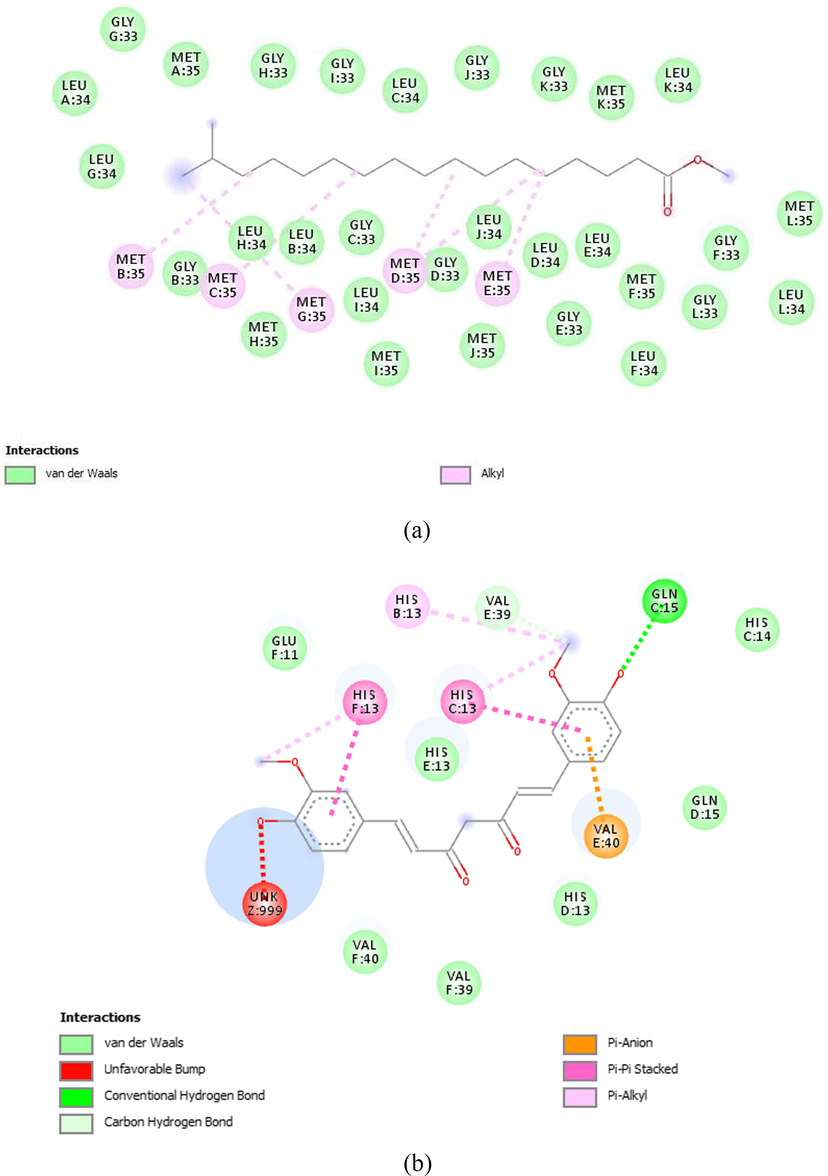
2D view of binding interaction: A. Heptadecanoic acid, 16-methyl-, methyl ester with 2LMN. B. Beta-sitosterol acetate with 3LII.
3.4 In silico ADME-Toxicity studies
Based on the docking study, the top five potential ligands of the selected extract were studied for their ADMET prediction includes intestinal absorption, blood–brain barrier (BBB) penetration, carcinogenicity and acute oral toxicity as shown in Table 1.
3.5 LigPlot analysis
LigPlot analysis was carried out to analyse hydrogen bond interaction and hydrophobic interactions of the in silico successful compounds were compared with standard which were shown in Table 2 and Fig. 4A–D. We found that the ligand heptadecanoic acid, 16-methyl-, methyl ester, with 2LMN interaction has 30 hydrophobic interactions and no H-bond interaction and similarly, the ligand 2-methyl-Z,Z-3,13-octadecadienol with 3LII is having 2 hydrophobic interactions, without any H-bond interactions, Figs. 68 and 69 shows the results of LigPlot analysis for these two lead phytoconstituents. Amino acid residues like Gly33(F), Gly33(H), Met35(L), Gly33(L), Leu34(F), Leu34(E), Met35(E), Leu34(J), Met35(F), Gly33(G), Met35(A), Leu34(B), Leu34(C), Leu34(D), Met35(B), Gly33(B), Gly33(C), Leu34(I), Leu34(D), Leu34(H), Gly33(J), Gly33(D), Gly33(E), Met35(D), Gly33(I), Leu34(G), Leu34(K), Met35(H), Met35(C), Leu34(L) were found to be forming molecular interactions heptadecanoic acid, 16-methyl-, methyl ester with 2LMN, where as 14 hydrophobic interactions Gly448(A), Ser203(A), Trp86(A), Phe338(A), Tyr337(A), Trp286(A), Arg296(A), Leu289(A), Ser293(A), Gln291(A), Glu292(A), Phe297(A), Tyr124(A), His447(A) and 1H-bond interaction Glu202(A) were observed with amino acid residues like beta-sitosterol acetate with 3LII.
S. No
Receptor
Compound No
Ligands
Binding site
1.
2LMN
49
Heptadecanoic acid, 16-methyl-, methyl ester
Gly33(F), Gly33(H), Met35(L), Gly33(L), Leu34(F), Leu34(E), Met35(E), Leu34(J), Met35(F), Gly33(G), Met35(A), Leu34(B), Leu34(C), Leu34(D), Met35(B), Gly33(B), Gly33(C), Leu34(I), Leu34(D), Leu34(H), Gly33(J), Gly33(D), Gly33(E), Met35(D), Gly33(I), Leu34(G), Leu34(K), Met35(H), Met35(C), Leu34(L).
–
Curcumin
Val 40(E), Glu11(F), His13(B), His13(F), Val39(E), Val40(F), His13(C), His13(D), Gln15(C), His13(E).
2.
3LII
60
Beta-sitosterol acetate
Gly448(A), Ser203(A), Trp86(A), Phe338(A), Tyr337(A), Trp286(A), Arg296(A), Leu289(A), Ser293(A), Gln291(A), Glu292(A), Phe297(A), Tyr124(A), His447(A), Glu202(A).
–
Galantamine
Arg478(A), Arg475(A), Asn482(A), Pro498(A), Asn490(A), Tyr479(A), Glu491(A).
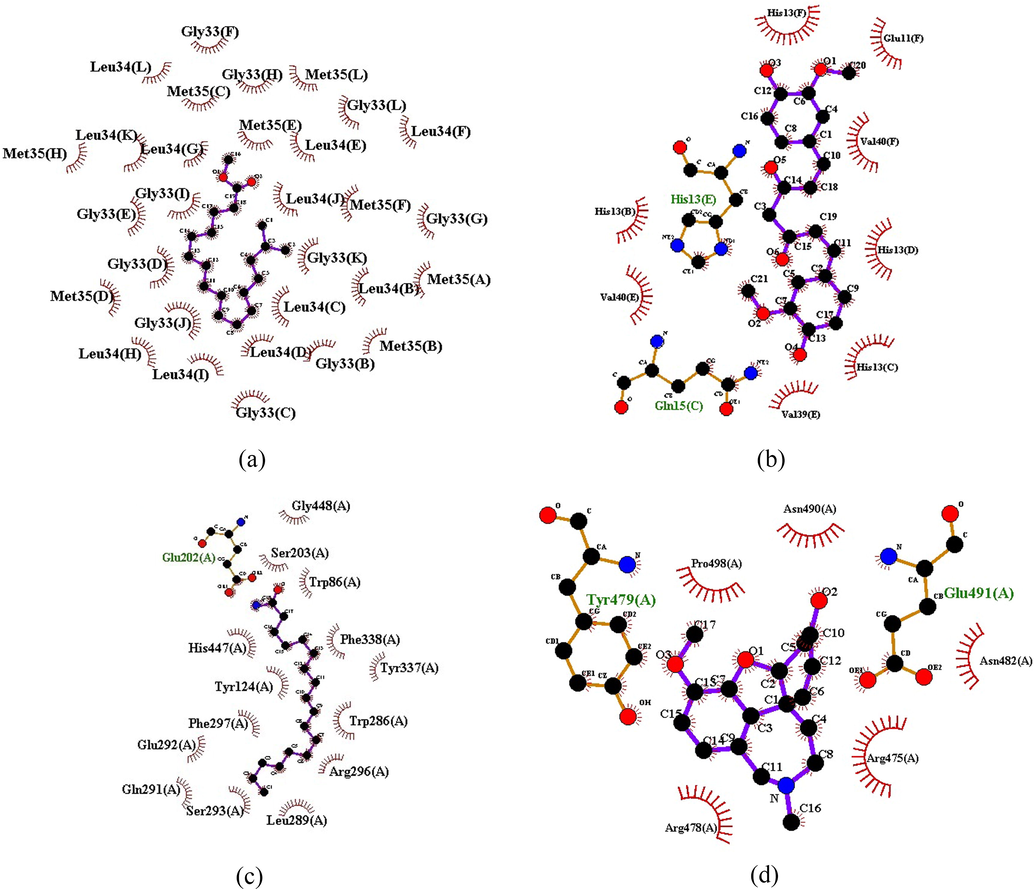
Ligplot analysis intermolecular interaction: A. Heptadecanoic acid, 16-methyl-, methyl ester with 2LMN. B. Curcumin with 2LMN. C. Beta-sitosterol acetate with 3LII. D. Galantamine with 3LII.
4 Discussion
Methanolic extract of N. cadamba has been subject to GC–MS/MS analysis and identified 61 phytoconstituents, 59 new phytoconstituents were detected. All these phytoconstituents were identified based on their different retention times and comparing of their mass spectral data with NIST database and Wiley library. Alzheimer's disease (AD) is a dynamic neurodegenerative issue with age-related symptoms, including cognitive impairment, psychosis, overactivity, aggressive behaviour, and depression (Hope et al., 1999). Aβ protein is present generally in the soluble form in the cerebrospinal fluid and blood (Seubert et al., 1992), however, in the patients with AD, Aβ gets fibrillized as the main constituent of amyloid plaques in the brain. The fibrillization of Aβ is considered as a main event in AD pathology, which cause to nerve cells death, cognitive and behavioural decline, is a one of the main characteristic feature of AD (Lorenzo and Yanker, 1994). Aβ aggregates of non-pathogenic proteins cause toxicity to neuronal cells (Bucciantini et al., 2002) and its clearance has not been fully understood yet (Citron, 2002). The identification of compounds that can defibrillize fibrillar Aβ or inhibit Aβ fibrillization are expected to be of therapeutic and preventive value in AD (Chauhan et al., 2004). AChE inhibitors retard the metabolic degradation of acetylcholine, optimising the availability of acetylcholine for communicating between cells (Moss et al., 2017). Aceytylcholinesterase inhibitors (AChEIs) are indicated for the treatment of mild-to-moderate AD (Trinh et al., 2003). There are only four medications that are approved by US-FDA namely donepezil, tacrina, galantamine and rivastigmine. Among them only donepezil was approved for the treatment of severe symptoms of AD (Mehta et al., 2012).
A total of 61 phytoconstituents were screened against two AD receptors (2LMN and 3LII) on the basis of their high binding affinity for the targets. We have identified 3 phytoconstituents are having least binding energy against Aβ inhibition, and 19 phytoconstituents where exhibited ACHE inhibition when compared with their standard curcumin and galantamine respectively. Top 5 phytoconsistuents have best Aβ and ACHE inhibitory activities are screen the further drug-likeness through the admetSAR prediction which reveals all the five compounds tested for Aβ inhibition heptadecanoic acid, 16-methyl-, methyl ester only one compound do not have carcinogenicity, and having better BBB permeation, intestinal absorption and less oral toxicity. Two out of five phytoconstistuents which are tested for ACHE inhibition activity are beta-sitosterol acetate and octadecanoic acid, 2-hydroxy-, methyl ester do not have any carcinogenicity and having better BBB permeation and less oral toxicity. These 3 compounds have shown highest binding affinity and better selectivity scores with both the therapeutic targets of AD.
5 Conculsion
We have identified novel phytoconstituents present in N. cadamba by GC–MS/MS studies and performed molecular docking studies against AD therapeutic targets. Thus our results theoretically support the anti AD effect of methanolic extract of N. cadamba. Hence our results prove that these phytoconstituents can be used to test their AD effect in vitro as well as in vivo to find out novel leads. The lead compounds can be further modified and optimized for better AD drugs development for novel anti Alzheimer’s drugs. The finding of this study may give researchers who are doing continuous efforts to develop new and effective formula to manage AD patients.
Acknowledgement
The authors are highly thankful to taxonomist Dr. Ravindrashukla, Assistant Professor, Department of Botany, Indira Gandhi National Tribal University, Amarkantak for plant authentication. In-charge Department of Nanotechnology, SRM University, Chennai, India for providing GC-MS/MS facility. The present research work is carried out by P.Subash, Research scholar under the guidance and supervision of Dr. Kareti Srinivasa Rao, Assistant Professor, Department of Pharmacy, Indira Gandhi National Tribal University, Amarkantak, India.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anthocephalus cadamba extract shows hypoglycemic effect and eases oxidative stress in alloxan–induced diabetic rats. Revista Brasileira de Farmacognosia.. 2011;21:155-164.
- [Google Scholar]
- Molecular docking of selected phytocompounds with H1N1 proteins. Bioinformation. 2015;11:196-202.
- [Google Scholar]
- Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives a new class of α-glucosidase inhibitors. J. Mol. Graph Model.. 2008;26:1202-1212.
- [Google Scholar]
- Anthocephalus alkaloids: Isodihydrocadambine. Tetrahedron Lett.. 1974;15:3335-3338.
- [Google Scholar]
- Inherent cytotoxicity of aggregates implies a common origin for protein misfolding diseases. Nature. 2002;416:507-511.
- [Google Scholar]
- Anti-inflammatory effect of the methanol extract from Anthocephalus cadamba stem bark in animal models. Int. J. Plant Biol.. 2010;1:30-32.
- [Google Scholar]
- Chauhan, N.K.C., Wegiel, W.J., Malik, M.N., 2004. Walnut extract inhibits the fibrillization of amyloid beta-protein and also defibrillizes its preformed fibrils current Alzheimer research. 1, 183–188.
- AdmetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model.. 2012;52:3099-3105.
- [Google Scholar]
- Emerging Alzheimer’s disease therapies: inhibition of β–secretase. Neurobiol. Aging. 2002;23:1017-1022.
- [Google Scholar]
- Small-molecule library screening by docking with PyRx. Chem. Biol. Springer 2015:243-250.
- [Google Scholar]
- Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;25:1403.
- [Google Scholar]
- Cadamba: a miraculous tree having enormous pharmacological implications. Pharmacogn. Rev.. 2015;9:107-113.
- [Google Scholar]
- β–Secretase as a therapeutic target for Alzheimer’s disease neurotherapeutics. J. Am. Soc. Exp. NeuroTherap.. 2008;5:399-408.
- [Google Scholar]
- Actions of bioactive phytochemicals in cell function and Alzheimer’s disease pathology. Micronutrients Brain Health. 2009;16:225-239.
- [Google Scholar]
- GC–MS analysis of phytochemical constituents in leaf extracts of Neolamarckia cadamba., rubiaceae. from Malaysia. Int. J. Pharm. Pharm. Sci.. 2014;6:123-127.
- [Google Scholar]
- Behaviour changes in Dementia 2: are there Behavioural syndromes ? Int. J. Geriat. Psychiatry.. 1997;12:1074-1078.
- [Google Scholar]
- Natural history of behavioural changes and psychiatric symptoms in Alzheimer’s disease: a longitudinal study. Br. J. Psychiatry. 1999;174:39-44.
- [Google Scholar]
- iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post–screening analysis. BMC Bioinf.. 2011;12:1-11.
- [Google Scholar]
- A quantitative histological study of early clinical and preclinical Alzheimer’s disease. Neuropathol. Appl. Neurobiol.. 1990;16:111-121.
- [Google Scholar]
- Efficacy of memantine in schizophrenic patients: a systematic review. J. Amino Acids 2017:1-6.
- [Google Scholar]
- Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharm. Biol.. 2001;39:74-79.
- [Google Scholar]
- Computational analysis of siRNA recognition by the Ago2 PAZ domain and identification of the determinants of RNA–induced gene silencing. PLoS ONE. 2013;8:2.
- [Google Scholar]
- Antihepatotoxic Effects of chlorogenic acid from Anthocephalus cadamba. Phytother. Res.. 1995;9:189-193.
- [Google Scholar]
- Khare, C.P., 2013. Indian Medicinal Plants In: Spriger reference. 53, 9.
- Krisnawati, H., Kallio, M., Kanninen, M., 2011. Anthocephalus cadamba Miq Ecology silviculture and productivity. 1–11.
- Ladokun, O.A., Abiola, A., Okikiola, D., Ayodeji, F., 2018. GC-MS and molecular docking studies of Hunteria umbellata methanolic extract as a potent anti–diabetic Informatics in Medicine Unlocked. 13, 1–8.
- Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta–analysis. Can. Med. Assoc. J.. 2003;169:557-564.
- [Google Scholar]
- A randomized placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology.. 2009;34:1322-1329.
- [Google Scholar]
- Β-Amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. USA. 1994;91:12243-12247.
- [Google Scholar]
- Beta-secretase as a target for Alzheimer’s disease drug discovery: an overview of in vitro methods for characterization of inhibitors. Anal Bioanal. Chem.. 2011;400:1979-1996.
- [Google Scholar]
- Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol.. 1999;48:471-480.
- [Google Scholar]
- New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis.. 2012;2012:1-8.
- [Google Scholar]
- ChemDraw 8 ultra windows and macintosh versions. J. Chem. Inf. Model.. 2004;44:2225-2226.
- [Google Scholar]
- Cholinesterase inhibitor therapy in Alzheimer’s disease: The limits and tolerability of irreversible CNS-selective acetylcholinesterase inhibition in primates. J. Alzheimer’s Dis.. 2017;55:1285-1294.
- [Google Scholar]
- Sedative and antiepileptic effects of Anthocephalus cadamba Roxb in mice and rats. Indian J. Pharmacol.. 2011;43:699-702.
- [Google Scholar]
- Adavances in selected medicinal and aromatic plants indigenous to Africa. J. Med. Plant Res.. 2009;3:86-95.
- [Google Scholar]
- Virtual screening and molecular docking analysis of Zap-70 kinase inhibitors. Int. J. Chem. Anal. Sci.. 2011;2:1208-1211.
- [Google Scholar]
- Traditional uses phytochemistry and pharmacological properties of Neolamarckia cadamba: a review. J. Ethnopharmacol. 2016:118-135.
- [Google Scholar]
- Prince, M., 2009. World Alzheimer Report. 1–96.
- Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1M HCl media. Corros. Sci.. 2013;69:292-301.
- [Google Scholar]
- Triterpene glycosides from the bark of Anthocephalus cadamba. J. Chem. Res. 2000:22-23.
- [Google Scholar]
- Ethnopharmacological survey of medicinal herbs in Israel the Golan Heights and the West Bank region. J. Ethnopharmacol.. 2002;83:251-265.
- [Google Scholar]
- Isolation and quantification of soluble Alzheimer’s β–peptide from biological fluids. Lett. Nat.. 1992;359:325-327.
- [Google Scholar]
- Inhibition of Alzheimer’s amyloid-beta aggregation in-vitro by carbenoxolone: Insight into mechanism of action. Neurochem. Int.. 2017;108:481-493.
- [Google Scholar]
- Elucidating the precise interaction of reduced and oxidized states of neuroglobin with Ubc12 and Cop9 using molecular mechanics studies. Int. J. Fundam. Appl. Sci.. 2012;1:74-77.
- [Google Scholar]
- Progress in the discovery of BACE inhibitors. Curr. Pharm. Des.. 2005;11:3383-3404.
- [Google Scholar]
- Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease. J. Am. Med. Assoc.. 2003;289:210-216.
- [Google Scholar]
- Antimicrobial wound healing and antioxidant activities of Anthocephalus cadamba. Afr. J. Tradit. Complement. Altern. Med.. 2007;4:481-487.
- [Google Scholar]
- Molecular docking and quantum mechanical studies on pelargonidin-3-glucoside as renoprotective ACE inhibitor. ISRN Comput. Biol. 2013:1-4.
- [Google Scholar]
- Evaluation of anti–pyretic activity of anthocephalus cadamba Roxb leaves extracts. Res. J. Pharm. Biol. Chem. Sci.. 2012;3:825-834.
- [Google Scholar]
- Human β–secretase BACE and BACE inhibitors: progress report. Curr. Top. Med. Chem.. 2006;6:569-578.
- [Google Scholar]
- The-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res. Ther.. 2011;3:1-6.
- [Google Scholar]
- Memantine augmentation in clozapine-refractory schizophrenia: a randomized double-blind placebo-controlled crossover study. Psychol. Med.. 2016;469:1909-1921.
- [Google Scholar]
- Evaluation of anti-inflammatory analgesic and antipyretic properties of Neolamarckia Cadamba on Wistar Albino Rats. J. Pharmacol. Clin. Res.. 2019;7:1-5.
- [Google Scholar]
- Ligplot: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Select.. 1995;8:127-134.
- [Google Scholar]
- Inhibition of delta-secretase improves cognitive functions in mouse models of Alzheimer’s disease. Nat. Commun. 2017:1-17.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.05.035.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2
Supplementary data 3
Supplementary data 3
Supplementary data 4
Supplementary data 4
Supplementary data 5
Supplementary data 5







