Translate this page into:
Optimized adsorption and effective disposal of Congo red dye from wastewater: Hydrothermal fabrication of MgAl-LDH nanohydrotalcite-like materials
⁎Corresponding author. mgoda199@gmail.com (Mohamed A. Farghali), mohamedfarghaly_p@sci.asu.edu.eg (Mohamed A. Farghali),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
MgAl-LDH was synthesized ideally using the hydrothermal method. MgAl-LDH exhibited excellent adsorption capacity for CR dye compared with previously prepared LDH compounds and other sorbents. The experimental adsorption data adequately fit the Langmuir isotherm model. Pseudo-second-order kinetic model best represents the adsorption data. The interaction mechanism of CR to the MgAl-LDH surface was strengthened by electrostatic attraction.
Abstract
The effectiveness of Congo red (CR) adsorption from aqueous solutions onto MgAl-layered double hydroxide (MgAl-LDH) nanosorbents was examined in this study. MgAl-LDH was synthesized using the hydrothermal method, and physicochemical characterization was performed via powdered X-ray diffraction, high-resolution transmission electron microscopy, Fourier transform infrared analysis, and zeta potential measurements. For optimum adsorption of CR onto the synthesized MgAl-LDH nanosorbent, the adsorption process was employed in batch experiments. Adsorption parameters, such as the adsorbent dosage, solution pH, contact time, and initial adsorbate concentration, vary with the adsorption kinetics and isotherm mechanism. The results of the batch experiments indicated rapid adsorption of CR dye from aqueous solutions onto MgAl-LDH during the first 30 min until equilibrium was achieved at 180 min with a dye concentration of 50 mg/100 mL and MgAl-LDH adsorbent dosage of 0.05 g. The experimental adsorption data fit adequately with the monolayer coverage under the Langmuir isotherm model (R2 = 0.9792), and showed the best fit with the pseudo-second-order kinetic model (R2 = 0.996). The change in zeta potential confirmed the effective adsorption interaction between the positively charged MgAl-LDH and the negatively charged CR molecules with electrostatic interactions. This work is distinguished by the successful hydrothermal preparation of MgAl-LDH in the form of homogenous nanoscale particles (∼100 nm). The prepared MgAl-LDH showed a high adsorption capacity toward anionic CR dye with a maximum adsorption capacity of 769.23 mg/g. This capacity is higher than those reported for other adsorbents in previous research.
Keywords
Layered double hydroxide
Adsorption
Congo red
Langmuir isotherm
Pseudo-second-order kinetic
1 Introduction
A major source of industrial pollution is wastewater contaminated with organic dyes owing to their intensive use in several applicable industries, such as paper, textiles, printing, solar cells, plastics, and cosmetics (Wang, 2019; Zhu, 2022). The high toxicity, low biodegradability, and high chemical stability of these organic dyes cause serious problems when they are not disposed of properly (Zhang, 2016; Lan, 2022). In addition, the accumulation of these organic dyes in water streams has negative effects on human health and aquatic life due to their mutagenic and carcinogenic effects (Quan, 2019; Verma, 2022). Congo red (CR) is an anionic pollutant azo dye with a complex aromatic structure. It is non-biodegradable, highly soluble in water, and highly resistant to traditional degradation. Because it has carcinogenic properties, as well as being harmful to the skin, eyes, respiratory system, and reproductive system, it is very important to remove CR dye from wastewater before it can reach natural water bodies (Mittal, 2009; Liu, 2022; Arab et al., 2022). Different wastewater treatment techniques include ion exchange, photochemical degradation, precipitation, membrane filtration, electrochemical oxidation, ozonation, coagulation/flocculation, and biological treatment (Goddeti, 2020; Eltaweil, 2021; Madan, 2019). Adsorption technology is among the most important and simple techniques because it is inexpensive and has low energy consumption and high disposal efficiency (Raval, 2016; Hu, 2018). The effectiveness of the adsorption process depends on the nature of the sorbent. Thus, the choice of adsorbent is a vital factor in enhancing the adsorption process and its efficiency (Hu, 2018). The ideal adsorbent is efficient, low-cost, stable, and environmentally friendly (Zhang, 2018). In combination with adsorbent materials, the role of nanotechnology is to change the sorbent properties and morphology by increasing the adsorbent surface area, thus increasing the adsorption capacity and pollutant-removal efficiency (Madan, 2019). Clay comprising layered double hydroxides (LDHs) is anionic nature and expressed as [M2+1−xM3+x(OH)2]x+[An−x/n]x−·mH2O, where M2+, M3+, and An− indicate divalent metal cations, trivalent metal cations, and interlayer anions, respectively, and × represents the molar ratio of M3+ to the total metal, which ranges from 0.2 to 0.33 for pure LDH structures (Deng, 2018; Woo, 2011; Xie et al., 2021). Promising applications of LDH in the field of anion adsorption are attributed to the multilayer structure of LDH, which comprises positively charged layers with high surface area, high porosity, good interlayer anionic exchangeability, and low cost of production (Xie et al., 2021; Baliarsingh et al., 2013; Goh et al., 2008). Several types of LDHs have been prepared and applied as adsorbents to remove different contaminants from wastewater (Goh et al., 2008; Theiss, 2014; Jawad, 2019; Mittal, 2021). Herein, preparation, full characterization of MgAl-LDH nanohydrotalcite and studying the different parameters affecting the adsorption capacity of LDHs toward CR dye, such as the contact time, initial dye concentration, pH of the solution, and adsorbate.
2 Experimental work
2.1 Materials
Aluminum nitrate nonahydrate (Al(NO3)3·9H2O), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), sodium hydroxide (NaOH), CR dye (C32H22N6Na2O6S2), and decarbonated water were purchased from Alfa Aesar (Germany) and used without further purification.
2.2 Synthesis of MgAl-LDH
The salts of Al(NO3)3·9H2O (0.5 M) and Mg(NO3)2·6H2O (1.0 M) were mixed together at a Mg2+:Al3+ ratio of 2:1, dissolved in 40 mL bidistilled water, and placed in a separatory funnel fixed in a one-gas inlet–outlet apparatus. A 2.0-M NaOH solution was prepared separately and placed in a three-neck round-bottom flask. Both the magnesium–aluminum and sodium hydroxide solutions were degassed by purging nitrogen gas. A salt solution of Mg(NO3)2·6H2O and Al(NO3)3·9H2O was added dropwise to the NaOH solution with vigorous stirring at 1500 rpm without heating for 6 h. The pH was held constant between 10 and 11 by adding 5 mL NaOH (2.0 M) every half hour. The white gelatinous precipitate of MgAl double-layer hydroxide was obtained through centrifugation at 12000 rpm at room temperature (25 °C) for 2 min and was perfectly washed several times with distilled water to eliminate any impurities. The white slurry was resuspended in 100 mL distilled water in a 250-mL Teflon–stainless reactor, heated for 14 h at 80 °C, and then separated by centrifugation. The slurry was dried at 70 °C for 48 h. The synthesis procedure for MgAl-LDH is depicted in Fig. 1.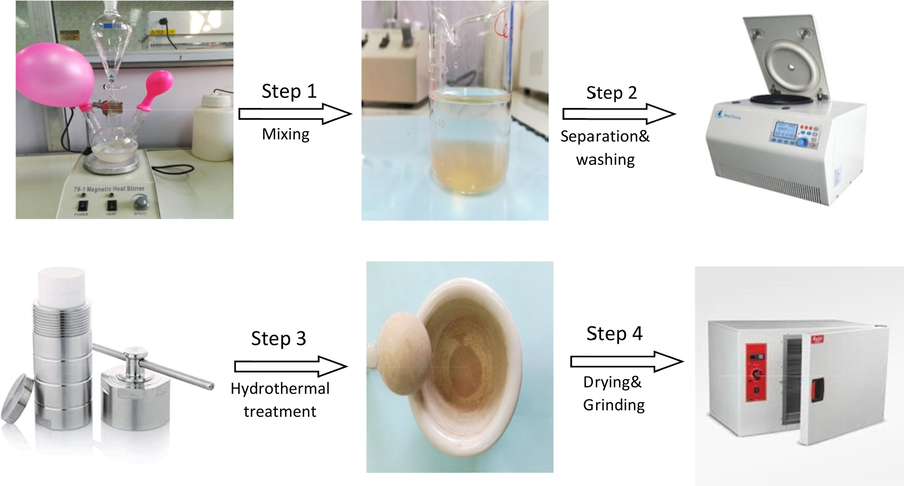
Schematic procedure for the synthesis of MgAl-LDH.
2.3 Characterization of LDH nanoparticles
The morphology of the synthesized LDH was examined via high-resolution transmission electron microscopy (HR-TEM). The captured images were recorded with JEOL JEM-100 using an accelerating voltage of 200 kV. The powder X-ray diffraction (PXRD) patterns of LDH were obtained using X’Pert PRO Panalytical with Cu Kα radiation (λ = 1.5406 Å). The diffraction patterns were acquired as a value of 2θ in the scanning range of 4–80 with a scanning rate of 2.4°/min. The functional group and intact structure of the LDH were recognized by Fourier transform infrared spectroscopy (FT-IR, Frontier MIR/FIR spectrometer; Perkin Elmer) with a scanning range from 4000 to 400 cm−1 using the conventional dried KBr disc. The surface charge or zeta potential was inspected using a Zetasizer Nano ZS analyzer, where 1 mg of the powder was dispersed in 10 mL of distilled water.
2.4 Adsorption study of Congo red
CR adsorption to MgAl-LDH was performed at room temperature via batch experiment under varying adsorption conditions. For the adsorption kinetics and isotherm determination, the contact time ranged from 1 to 180 min, and 0.05 g of the sorbent was added to 100 mL of CR solutions with concentrations varying between 50 and 1000 mg/L and shaken using a benchtop shaker (Unimax 1010 DT; Heidolph, Germany; 350 rpm). The pH of the CR dye solution was also varied between 3 and 11 during the adsorption experiment, and the sorbent dose was changed from 0.01 to 0.2 g. The efficiency of the adsorption process was detected by measuring the color intensity of the remaining CR concentration using ultraviolet-visiblenear-infrared spectrophotometry (UV–vis-NIR; Cary 5000; Varian, UK) at λmax = 498 nm of CR after preparing the calibration curve based on standard CR concentrations. The equilibrium adsorption capacity qe (mg/g) was defined as the amount of adsorbed CR (mg) per unit mass of the sorbent (g) and can be determined by Equation (1) (Yan, 2017).
The removal efficiency percentage (R%) was obtained from Equation (2) (Jinhua, 2010).
2.5 Adsorption data analysis
2.5.1 Effect of contact time and kinetics study
The kinetics study is required to express the adsorption rate of the contaminant (CR) on the surface of the sorbent (MgAl-LDH) to determine the residence time at the liquid–solid interface, determine the sorption mechanism, and determine the proper sorption treatment process (Qiu, 2009). Three adsorption kinetics models were employed to investigate the adsorption behavior: the pseudo-first-order, pseudo-second-order, and Elovich kinetic models. The correlation coefficients effectively describe the applicability of the corresponding kinetic models. The pseudo-first-order equation is (Simonin, 2016).
The pseudo-second-order kinetic model is used to determine the adsorption of a single layer of solute (adsorbate) on a solid particle (adsorbent) without interactions between the sorbed species and is expressed as follows (Simonin, 2016; Faisal, 2021):
The Elovich model equation is generally represented by (Wang et al., 2009).
The straight line obtained from the relation between qt and ln t provides the Elovich constants β and α from its slope and intercept, respectively.
2.5.2 Effect of initial adsorbate concentration and adsorption isotherms models
The experimental adsorption data are expressed mathematically using isotherm models to describe the relation between the initial concentration and adsorption capacity when the adsorbate is initially distributed at the equilibrium state between the solid and liquid phases. The adsorption capacity increases nonlinearly with increasing initial concentration (Zheng, 2009). Three adsorption isotherm models were used to fit the adsorption data, namely, the Langmuir, Freundlich, and Temkin isotherm models.
The Langmuir isotherm model can describe the monolayer coverage at specific homogeneous adsorbent sites without interaction between the chemical species adsorbed on the neighboring areas (Faisal, 2021; [32]). The simple form of the Langmuir isotherm model is represented as (Dehghani, 2016).
The Freundlich adsorption isotherm model is associated with the hypothesis that multilayer adsorption exists on heterogeneous adsorbent surfaces (Bharali and Deka, 2017), on which the active sites are not energetically uniform. The Freundlich model is expressed by Equation (8) (Yang, 2011).
The Temkin isotherm model considers the presence of adsorbent–adsorbate interaction and assumes that the heat adsorption of all molecules in the layer decreases linearly with surface coverage. A linear decrease in adsorption energy as the adsorption sites are filled is also assumed, as well as a uniform distribution of binding energies up to a maximum value. The linear form for this model is (Lafi, 2016).
2.5.3 Effect of pH and sorbent dosage
The pH is a significant parameter reflecting the nature of the solid–liquid adsorption interaction and can affect the adsorbate’s degree of ionization and the adsorbent’s surface charge (Lafi, 2016). The effect of pH on the adsorption capacity of MgAl-LDH was investigated using CR solution with pH ranging from 3 to 11, adjusted by pH meter (Orion Versa Star; Thermo Scientific, USA) by dropwise addition of HCl (0.1 M) or NaOH (0.1 M).
The effect of the mass of MgAl-LDH on its adsorption of CR from aqueous solution was investigated using 100 mL of 500-mg/L CR solutions with the mass of sorbent varying from 0.01 to 0.2 g.
3 Results and discussion
3.1 Characterization of MgAl-LDH
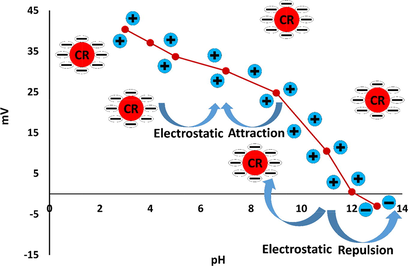
The phase-determination results of free MgAl-LDH and MgAl-LDH intercalated with CR dye (MgAl-LDH-CR) are shown in Fig. 2. The PXRD diffraction patterns demonstrate that MgAl-LDH was perfectly synthesized with high crystallinity of the pure hydrotalcite phase, based on the presence of strong peaks located at 2θ = 11.24°, 22.68°, 34.34°, 38.19°, 45.17°, 60.30°, and 61.61° corresponding to reflection planes (hkl) of (0 0 3), (0 0 6), (0 1 2), (0 1 5), (0 1 8), (1 1 0), and (1 1 3), respectively, corresponding to the MgAl-LDH carbonate standard ICCD card (04–015–4253). The diffraction pattern of MgAl-LDH-CR after the adsorption process to show the peaks as free MgAl-LDH with multiple additional weak peaks is attributed to the presence of CR molecules intercalated to the MgAl-LDH surface. This indicates very good adsorption of CR via attachment to the layers and planes of the LDH. The peaks related to CR molecules are positioned at 2θ = 4.54°, 15.55°, 18.36°, 20.45°, 25.66°, 26.83°, 28.55°, and 31.66°, corresponding to the CR reference card (00–036–1788). Furthermore, the diffraction pattern of CR-loaded MgAl-LDH exhibited peak broadening of the LDH crystal structure, which could be caused by the exchange of water molecules with CR molecules between the layers spacing of the LDH structure. This causes increased d-spacing of the LDH and, therefore, widens the peaks and decreases the crystal size of MgAl-LDH (Dasgupta, 2017). The FT-IR results, Fig. 3. are inconsistent with the PXRD results. In the case of the unloaded MgAl-LDH, a broadened band extended from 3300 to 3600 cm−1 that was attributed to the OH stretching vibration of water molecules present in the spacing between the LDH layers and metal hydroxides (Shabanian et al., 2020). A significant band approximately 1637 cm−1 is related to OH bending vibration. Other characteristic absorption bands are noted in the range of 450–1100 cm−1, attributed to metal–oxygen and oxygen–metal–oxygen bands, including one at exactly 452 cm−1 due to the Al–O bond in (AlO6)−3 (Valcheva-Traykova et al., 1993). For MgAl-LDH-CR, all the absorption bands present in MgAl-LDH were also present, with the addition of bands related to the CR dye. A band related to the characteristic vibration stretching of the mono azo group (–N⚌N–) extended from 1500 to 1550 cm−1. The peak at 1624 cm−1 is typical for red azo dyes. Furthermore, the weak bands at 1167 cm−1 and 1369 cm−1 are attributable to the asymmetrical stretching vibration of the S—O(SO3–H) group and nitro group (NO2). The morphological properties were examined using HR-TEM. According to Fig. 4A and 4B, the MgAl-LDH sorbent forms well-structured hexagonal nanostructures with sizes approximately 100 nm. In addition, the presence of the small bulky aggregations in MgAl-LDH-CR represented in Fig. 4B related to the accumulation of CR dye confirming the high absorptivity of LDH toward CR molecules on its surface, as well as the ion exchange of CR with the water molecules present between the LDH layers (Dasgupta, 2017). Zeta potential analysis was employed to characterize the surface charges of LDH at different pH values in the range between 3 and 13 to determine the point of zero charges (pHpzc) value of MgAl-LDH, as shown in Fig. 5. It was clearly observed that the pHpzc of MgAl-LDH is 12.0. At a pH lower than 12.0, the surface of the MgAl-LDH has positive charges, whereas at higher pH values, it has negative charges that can enhance the adsorption (Jia, 2019).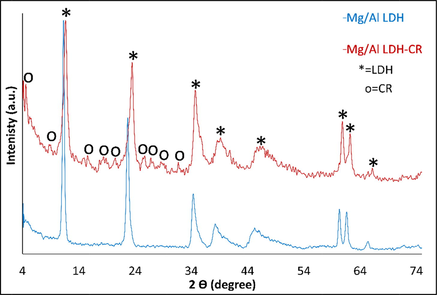
PXRD Diffraction pattern of free MgAl-LDH and loaded LDH intercalated CR.
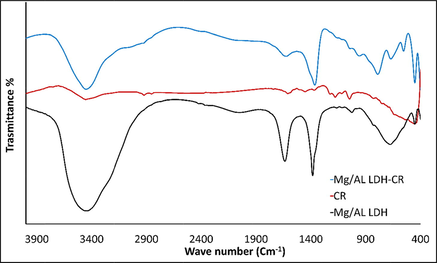
FT-IR of free MgAl-LDH and loaded LDH intercalated CR and pure CR dye.
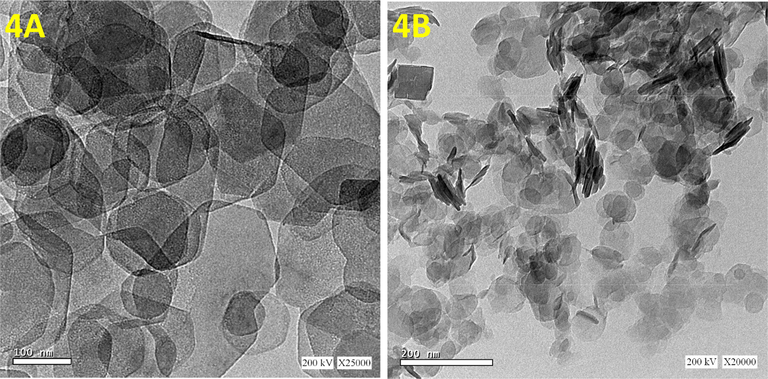
HRTEM images of free MgAl-LDH (4A) and loaded LDH intercalated CR (4B).
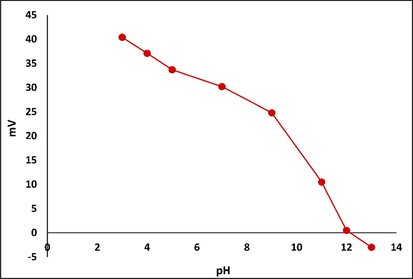
Zeta potential measurements of the MgAl-LDH in aqueous solution with the variation of the pH values.
3.2 Adsorption efficiency of MgAl-LDH
3.2.1 Effect of contact time and kinetics study
The adsorption capacity of MgAl-LDH under varying contact time for the removal of CR is shown in Fig. 6A; the adsorption capacity increases with increasing reaction time, thus enhancing the removal of the CR contaminant from the aqueous solution until equilibrium is reached. The adsorption of CR was very fast in the first 30 min. After that, the adsorption gradually increases, indicating that the equilibrium state was reached after 180 min. The first stage is rapid due to surface complexation or electrostatic sorption onto the adsorbent. In contrast, weak sorption occurred in the second stage due to the insufficient free sorption active sites on the surface of MgAl-LDH (Ahmed, 2020).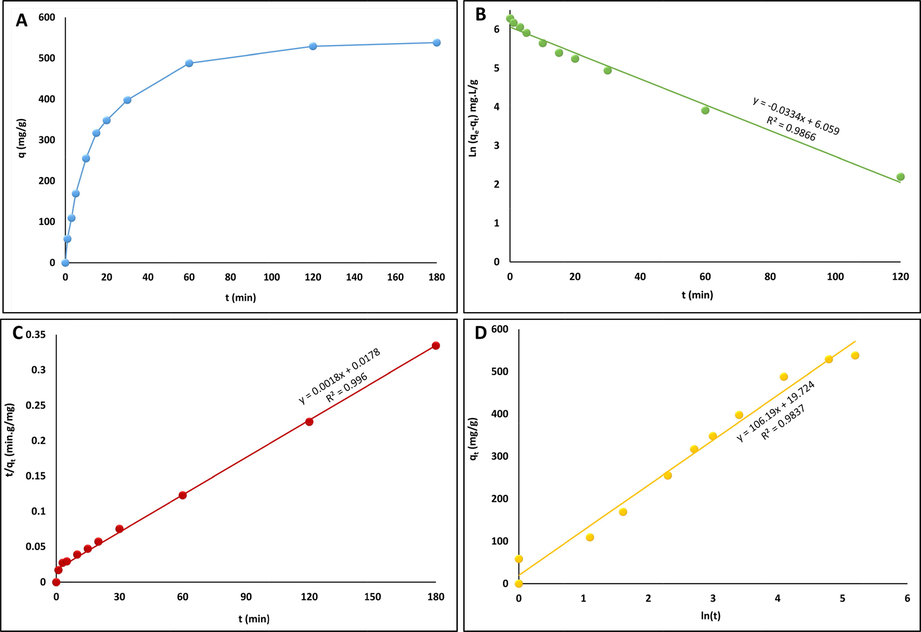
Effect of contact time on the adsorption of CR onto MgAl–LDH (Co = 500 mg/L, m = 0.05 g/100 mL) (A), and linear fitting adsorption experimental data of CR onto MgAl–LDH using pseudo-first-order (B), pseudo-second-order (C) and Elovich kinetic models (D).
The adsorption kinetics were studied by analyzing the experimental data with pseudo-first-order, pseudo-second-order, and Elovich kinetic models, as illustrated in Fig. 6B–6D. The kinetic parameters for adsorption of CR onto MgAl-LDH for the different kinetic models are listed in Table 1. Depending on the values of the calculated correlation coefficients (R2), the pseudo-second-order model is much closer than the pseudo-first-order and Elovich kinetic models to describing the behavior of CR adsorption on MgAl-LDH (Taher, 2021). Furthermore, the values of the calculated equilibrium adsorption capacity (qe) and the experimental adsorption capacity of the pseudo-second-order model are very close, which further proves the applicability of the pseudo-second-order model for predicting the adsorption process controlled by chemisorption (Sriram, 2020), in which the adsorption process is defined by electron exchange or sharing between the adsorbent and adsorbate (Miao, et al., 2021). Generally, LDHs possess a dual-charge nature, with positively charged brucite-like layers and negative interlayer anions, such as hydroxide ions. Consequently, the interaction between LDHs and CR is mainly dependent on the electrostatic interactions between the anionic CR and the positively charged LDH metal layers (Wang, 2018).
Adsorbate
Pseudo-first-order model
Elovich model
qCalc [mg/g]
K1 [min−1]
R2
β [g/mg]
α [mg/g.min]
R2
CR
427.95
0.0334
0.9866
0.0094
223.24
0.9837
Adsorbate
Pseudo-second-order model
K2 [g/mg.min]
qCalc [mg/g]
qExp [mg/g]
h [mg/g.min]
R2
CR
0.00018
555.56
528.0
56.18
0.996
3.2.2 Effect of initial adsorbate concentration and adsorption isotherms models
The adsorption isotherms of MgAl-LDH were used to examine the relation between the adsorption capacity of the sorbent at different initial concentrations of adsorbate at room temperature that give important data for evaluating the adsorption system (Zhang, 2018). In Fig. 7A, it can be noted that with increasing initial concentration of CR solution, the adsorption capacity of MgAl-LDH increased sharply with a maximum adsorption capacity of 767.8 mg/g at 1000 mg/L CR. This phenomenon is attributed to the ratio between adsorbate species and the number of free active sites being small at low adsorbate concentrations, causing mass transfer resistance between the adsorbate aqueous solution and the adsorbent and leading to decreased adsorption capacity. In contrast, a higher initial concentration of adsorbate enhances the driving force beyond the mass transfer resistance of the adsorbate species, thus increasing the adsorption capacity of the sorbent (Aksu and Tezer, 2005; Farghali et al., 2021).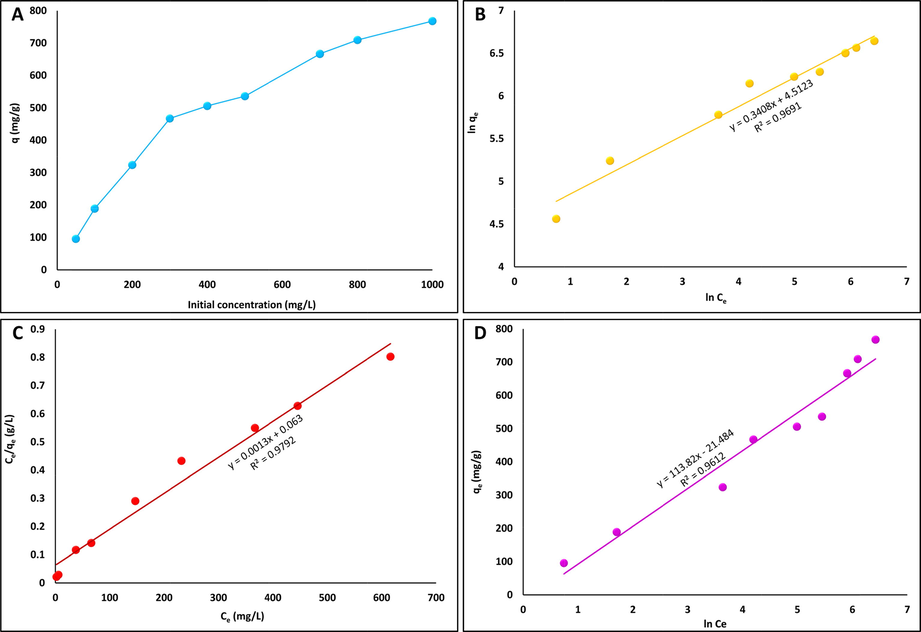
Effect of initial CR concentrations (100–1000 mg/L) on adsorption capacity of MgAl–LDH for the removal of CR from aqueous solution (contact time = 120 min, m = 0.05 g/100 mL) (A), Linear fitting curves of the experimental adsorption data with the Freundlich isotherm model (B), Langmuir isotherm model (C) and Temkin isotherm model (D) for adsorptive removal of CR from aqueous solution using MgAl–LDH.
To further investigate the interaction between the adsorbate and adsorbent, the experimental adsorption data were analyzed using the Freundlich, Langmuir, and Temkin adsorption isotherm models, as shown in Fig. 7B–7D, and the calculated parameters are recorded in Table 2. The Freundlich model assumes that heterogeneous adsorption sites are present on the surface of the adsorbent, and that the adsorbed species mutually affect one another. In addition, adsorption in this model is not bound to the single layer (Zheng, 2018). However, the Langmuir model assumes that the adsorbed particles are mutually independent and tied in a single layer with the adsorbent surface. The Temkin model assumes that adsorbent–adsorbate interaction, and therefore the heat adsorption, decreases linearly with coverage for all molecules in the layer (Lafi, 2016). Compared with the Freundlich and Temkin models, the Langmuir isotherm model showed the best fit for the experimental adsorption data of CR on MgAl-LDH with a correlation coefficient value (R2 = 0.9792) greater than those of the Freundlich and Temkin models (R2 = 0.9691 and 0.9612, respectively), indicating a monolayer adsorption process in which only a single layer of CR molecules can be adsorbed onto the surface of the MgAl-LDH sorbent. qm of the MgAl-LDH is calculated from the Langmuir isotherm to be 769.23 mg/g, which is very close to the experimental value of 767.8 mg/g, providing further confirmation that the Langmuir isotherm model is a good fit for the adsorption data. According to Table 2, the calculated RL values are within 0.046–0.492, indicating the favorability of the adsorption process. The lower value of the Langmuir constant (b) indicates CR's affinity toward MgAl-LDH.
Adsorbate
Freundlich isotherm
Temkin isotherm
n
kf
R2
B
bT [g/mol]
AT [L/g]
R2
CR
2.93
91.13
0.9691
113.82
21.77
0.828
0.9612
Adsorbate
Langmuir isotherm
qmax [mg/g]
B [L/mg]
RL
R2
CR
769.23
0.0206
0.492–0.046
0.9792
Furthermore, the favorability of the adsorption process can be confirmed from the value of the Freundlich heterogeneity factor (1/n); in Table 2, 1/n = 0.34 (Vimonses, 2009). A comparison of the previously recorded adsorption capacities of various sorbents for the removal of CR and that found in this work is listed in Table 3.
Adsorbent
CR adsorption capacity, qe [mg/g]
Reference
RGO/NH2-MIL-68(Al)
473.9
(Wu, 2017)
MNPs@NiFe LDH
79.6
(Taher, 2021)
Mesoporous activated carbon
14.2
(Mandal, 2021)
Micro-/nanostructured NiO microspheres
456.8
(Jia, 2021)
Mn-UiO-66@GO-NH2 composite
1265.8
(Eltaweil, 2021)
Hierarchical porous ZnO
334.0
(Lei, 2017)
Fe3O4@ZTB-1
458.0
(Han, 2019)
SBPF
417
(Zhang, 2016)
Gum ghatti acrylamide-grafted copolymer coated with zero-valent iron
153.3
(Goddeti, 2020)
Bentonite
158.7
(Lian et al., 2009)
ZnO@Ze composite
161.3
(Madan, 2019)
Chitosan/organo-montmorillonite
293.3
(Wang, 2007)
ZnO/SnO2 heteronanofibers
85.8
(Chen, 2015)
Powdered egg shell
96.0
(Zulfikar and Setiyanto, 2013)
MgAl-LDH
769.23
[This work]
3.2.3 Effect of sorbent dosage and pH
The amount of adsorbent added to the aqueous solution of contaminant significantly affects the removal efficiency of the adsorbate molecules during the adsorption process. Fig. 8. illustrates the effect of varying the amount of MgAl-LDH between 0.01 and 0.2 g/100 mL to remove CR from aqueous solution. A significant enhancement in CR removal from 14.8 % to 100.0 % is observed with increasing MgAl-LDH dose from 0.01 to 0.2 g/100 mL. The number of available adsorption active sites in aqueous solution increased with increasing adsorbent dose, thus increasing the probability of removing the contaminants from the aqueous solution. In contrast, the adsorption capacity of MgAl-LDH decreased with further increases in adsorbent dosage because the dispersion of adsorbent particles is uniform in aqueous solution for a specific adsorbent dosage range. All the available active sites are often entirely exposed, which enhances the accessibility of CR molecules onto a larger number of active sites. However, a further increase in adsorbent dosage leads to an increase in the number of adsorption active sites with higher energy, which could cause a decrease in a more significant fraction of active sites with lower energy, thus decreasing the adsorption capacity (Zubair, 2017). Photograph of the effect of MgAl-LDH dose on the adsorption capacity and removal efficiency of CR is shown in Fig. 9.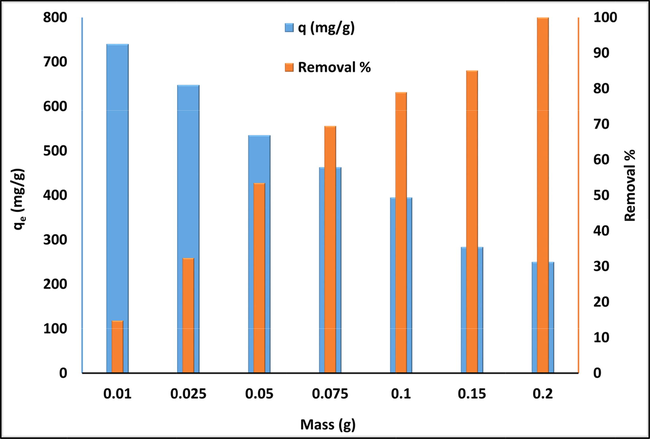
Effect of MgAl–LDH dose on the adsorption capacity and removal efficiency of CR, (contact time = 120 min, Co = 500 mg/L).

Effect of MgAl–LDH dose photograph on CR's removal efficiency. (Contact time = 120 min, Co = 500 mg/L, sorbent dose varying between 0.01 and 0.2 g/100 mL).
The pH is considered a significant factor affecting the adsorption process because of its influence on the surface functionalization of the solid adsorbent and the degree of ionization/dissociation of the adsorbate particles in the aqueous solution (Jia, 2019; Guan, 2017). The influence of pH on the adsorption capacity of MgAl-LDH to remove CR from aqueous solution is shown in Fig. 10. The results could be predicted from the behavior of the MgAl-LDH zeta potential curve in Fig. 5. The highest uptake of CR dye occurs when pH < pHpzc, where pHpzc = 12.0. This means that the surface of the adsorbent is protonated for pH < 12 due to the release of hydrogen ions, which can enhance the adsorption capacity of the adsorbent for anionic dyes through the generation of a strong electrostatic attraction force between the positively charged solid adsorbent surface and the negative charges of the negatively charged dye solution. In the MgAl-LDH interlayer, OH−, HCO3−, or CO32− ions were substituted with the SO3− of the CR molecules via ion exchange (Li, 2016). With increasing pH of the CR solution, the positive charges on the surface of MgAl-LDH gradually decrease, and the negative charges increase; as a result, an acidic medium is needed to achieve the optimum adsorption conditions. As a consequence, the electrostatic attraction force between MgAl-LDH and the CR dye solution weakens gradually with increasing pH, resulting in a decrease in adsorption efficiency (Zheng, 2019). The effect of the solution pH photograph on CR adsorption by MgAl-LDH is presented in Fig. 11.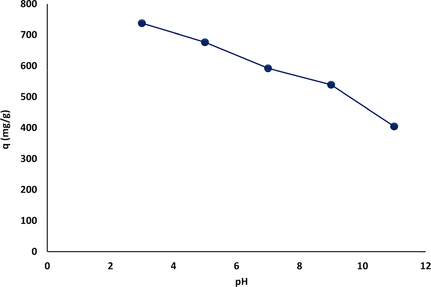
Effect of solution pH on CR adsorption by MgAl-LDH nanosorbent (contact time = 120 min, Co = 500 mg/L, m = 0.05 g, solution pH varying from 3 to 11).

Effect of solution pH photograph on CR adsorption by MgAl-LDH (contact time = 120 min, Co = 500 mg/L, m = 0.05 g, solution pH varying from 3 to 11).
3.2.4 Adsorption mechanism
The adsorption mechanism of the CR anionic dye by MgAl-LDH nanosorbent comprises two mechanisms: adsorption on the external surface and ion exchange. In the first approach, the adsorption of CR occurs on the outer surface of sorbent through electrostatic attraction and H-bonding (Li, 2016). The electrostatic attraction generated between the negatively charged sulfonate group of CR dye and the positively charged LDH surface below the point of zero charges depends on the value of the effectiveness of zero charges of MgAl-LDH, as shown in Fig. 5. H-bonding is favored between the hydroxyl groups of the LDH surface and negatively charged groups of CR dye (Chatterjee, 2007). The ion-exchange process entails inserting CR molecules and replacing the anions present in the interlayer spacing (CO32−, HCO3−, or OH−) with CR anions (Lei, 2017; Olfs, 2009).
4 Conclusions
The sorbent was successfully prepared using a hydrothermal method, and its physicochemical properties were fully characterized via XRD, HR-TEM, FT-IR, and zeta potential analysis. It was then applied as an effective nanosorbent to remove anionic CR dye from aqueous solution. Different sorbent parameters, such as contact time, initial CR concentration, sorbent dosage, and pH, were studied as effective parameters in the adsorption of CR dye over the surface of the MgAl-LDH nanosorbent. The adsorption mechanism was enhanced by electrostatic attraction and H-bonding between the negative charges of the anionic CR dye and the positive surface of the MgAl-LDH nanosorbent below the point of zero charge. The results of the experimental data were fitted using the pseudo-second-order kinetic model and Langmuir isotherm model, which suggest a monolayer adsorption process with a maximum adsorption capacity of 769.23 mg/g, which is higher than that found in previous works using MgAl-LDH for the removal of CR.
Acknowledgments
This work is partially funded by: This authors world like to thank the Science, Technology, and Innovation Funding Authority, Project title: “Eco-friendly Pesticides against Pests of Medical, Veterinary, and Agricultural Importance” ID: 41608 for partially supporting this study.
Funding
This work was partially supported by the Science, Technology, and Innovation Funding Authority, Project title: “Eco-friendly Pesticides against Pests of Medical, Veterinary, and Agricultural Importance” ID: 41608.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Scient. Rep.. 2020;10(1):1-12.
- [Google Scholar]
- Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem.. 2005;40(3–4):1347-1361.
- [Google Scholar]
- Arab, C., R. El Kurdi, and D. Patra, Zinc curcumin oxide nanoparticles for enhanced adsorption of Congo red: kinetics and adsorption isotherms study. 2022. 23: p. 100701
- Baliarsingh, N., K. Parida, and G.J.R.a. Pradhan, Influence of the nature and concentration of precursor metal ions in the brucite layer of LDHs for phosphate adsorption–a review. 2013. 3(46): p. 23865-23878.
- Bharali, D. and R.C.J.J.o.e.c.e. Deka, Adsorptive removal of congo red from aqueous solution by sonochemically synthesized NiAl layered double hydroxide. 2017. 5(2): p. 2056-2067.
- Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf. A: Phsicochem. Eng. Asp.. 2007;299(1–3):146-152.
- [Google Scholar]
- Chen, X., et al., The synthesis of ZnO/SnO 2 porous nanofibers for dye adsorption and degradation. 2015. 44(7): p. 3034-3042
- Controlled release of ibuprofen using Mg Al LDH nano carrier. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017.
- [Google Scholar]
- Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq.. 2016;215:671-679.
- [Google Scholar]
- Sodium dodecyl sulfate intercalated and acrylamide anchored layered double hydroxides: a multifunctional adsorbent for highly efficient removal of Congo red. J. Colloid Interfaces Sci.. 2018;521:172-182.
- [Google Scholar]
- Eltaweil, A.S., et al., Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. 2021. 379: p. 407-416
- Green synthesis for novel sorbent of sand coated with (Ca/Al)-layered double hydroxide for the removal of toxic dye from aqueous environment. J. Environ. Chem. Eng.. 2021;9(4):105342
- [Google Scholar]
- Farghali, M.A., M.M. Abo-Aly, and T.A.J.I.C.C. Salaheldin, Modified mesoporous zeolite-A/reduced graphene oxide nanocomposite for dual removal of methylene blue and Pb2+ ions from wastewater. 2021. 126: p. 108487
- Removal of Congo red from aqueous solution by adsorption using gum ghatti and acrylamide graft copolymer coated with zero valent iron. Int. J. Biol. Macromol.. 2020;149:21-30.
- [Google Scholar]
- Goh, K.-H., T.-T. Lim, and Z.J.W.r. Dong, Application of layered double hydroxides for removal of oxyanions: a review. 2008. 42(6-7): p. 1343-1368.
- A facile approach to synthesize 3D flower-like hierarchical NiCo layered double hydroxide microspheres and their enhanced adsorption capability. Colloids Surf. A: Phsicochem. Eng. Asp.. 2017;529:907-915.
- [Google Scholar]
- Han, L.-J., et al., Effective adsorption of Congo red by a MOF-based magnetic material. 2019. 48(14): p. 4650-4656
- Ho, Y.S., J.C.Y. Ng, and G. McKay, REMOVAL OF LEAD(II) FROM EFFLUENTS BY SORPTION ON PEAT USING SECOND-ORDER KINETICS. 2001. 36(2): p. 241-261.
- Ultra-high adsorption capacity of MgO/SiO2 composites with rough surfaces for Congo red removal from water. J. Colloid Interface Sci.. 2018;510:111-117.
- [Google Scholar]
- Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: different intercalated anions with different mechanisms. J. Clean. Prod.. 2019;211:1112-1126.
- [Google Scholar]
- Jia, Y.-H., et al., Preparation of borate anions intercalated MgAl-LDHs microsphere and its calcinated product with superior adsorption performance for Congo red. 2019. 575: p. 373-381
- Fast removal of Congo red from aqueous solution by adsorption onto micro/nanostructured NiO microspheres. Mater. Sci. Eng.: B. 2021;270:115228
- [Google Scholar]
- Rapid adsorption of Cr (VI) on modified halloysite nanotubes. Desalination. 2010;259(1):22-28.
- [Google Scholar]
- Adsorption study of Congo red dye from aqueous solution to Mg–Al–layered double hydroxide. Adv. Powder Technol.. 2016;27(1):232-237.
- [Google Scholar]
- Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: a review on species, mechanisms and perspectives. Chemosphere. 2022;293:133464
- [Google Scholar]
- Langmuir, I.J.J.o.t.A.C.s., The adsorption of gases on plane surfaces of glass, mica and platinum. 1918. 40(9): p. 1361-1403.
- Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci.. 2017;490:242-251.
- [Google Scholar]
- Organic dye removal from aqueous solutions by hierarchical calcined Ni-Fe layered double hydroxide: Isotherm, kinetic and mechanism studies. J. Colloid Interface Sci.. 2017;496:158-166.
- [Google Scholar]
- Li, B., et al., Different dye removal mechanisms between monodispersed and uniform hexagonal thin plate-like MgAl–CO32--LDH and its calcined product in efficient removal of Congo red from water. 2016. 673: p. 265-271
- Lian, L., L. Guo, and C.J.J.o.h.m. Guo, Adsorption of Congo red from aqueous solutions onto Ca-bentonite. 2009. 161(1): p. 126-131.
- Fabrication of CoFe-MOF materials by different methods and adsorption properties for Congo red. J. Mol. Liq.. 2022;360:119405
- [Google Scholar]
- Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl. Surf. Sci.. 2019;487:907-917.
- [Google Scholar]
- Mandal, S., et al., Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: Characterization, adsorption kinetics, and isotherm studies. 2021. 243: p. 103869
- Miao, J., et al., Feasible synthesis of hierarchical porous MgAl-borate LDHs functionalized Fe3O4@ SiO2 magnetic microspheres with excellent adsorption performance toward congo red and Cr (VI) pollutants. 2021. 861: p. 157974.
- Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci.. 2009;340(1):16-26.
- [Google Scholar]
- Mittal, J.J.J.o.E.M., Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review. 2021. 295: p. 113017.
- Olfs, H.-W., et al., Comparison of different synthesis routes for Mg–Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. 2009. 43(3-4): p. 459-464
- Qiu, H., et al., Critical review in adsorption kinetic models. 2009. 10(5): p. 716-724
- Polyethyleneimine (PEI) incorporated Cu-BTC composites: Extended applications in ultra-high efficient removal of congo red. J. Solid State Chem.. 2019;270:231-241.
- [Google Scholar]
- Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review. Environ. Sci. Pollut. Res.. 2016;23(15):14810-14853.
- [Google Scholar]
- FTIR characterization of layered double hydroxides and modified layered double hydroxides. In: Layered Double Hydroxide Polymer Nanocomposites. Elsevier; 2020. p. :77-101.
- [Google Scholar]
- On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J.. 2016;300:254-263.
- [Google Scholar]
- Sriram, G., et al., Mg–Al-layered double hydroxide (LDH) modified diatoms for highly efficient removal of Congo red from aqueous solution. 2020. 10(7): p. 2285
- Preparation of magnetite-nanoparticle-decorated NiFe layered double hydroxide and its adsorption performance for congo red dye removal. Chem. Phys. Lett.. 2021;777:138712
- [Google Scholar]
- A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci.. 2014;417:356-368.
- [Google Scholar]
- Valcheva-Traykova, M., N. Davidova, and A.J.J.o.m.s. Weiss, Thermal decomposition of Mg, Al-hydrotalcite material. 1993. 28(8): p. 2157-2162.
- M. Verma et al. Verma, M., et al., Multifunctional β-Cyclodextrin-EDTA-Chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. 2022. 292: p. 118447
- Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J.. 2009;148(2–3):354-364.
- [Google Scholar]
- Wang, L., et al., Removal of Congo red from aqueous solution using a chitosan/organo‐montmorillonite nanocomposite. 2007. 82(8): p. 711-720
- Wang, X., et al., Cotton fiber-supported layered double hydroxides for the highly efficient adsorption of anionic organic pollutants in water. 2018. 42(12): p. 9463-9471
- In situ growth of ZIF-8 nanoparticles on chitosan to form the hybrid nanocomposites for high-efficiency removal of Congo Red. Int. J. Biol. Macromol.. 2019;137:77-86.
- [Google Scholar]
- Wang, X.S., Z.Z. Li, and S.R. Tao, Removal of chromium (VI) from aqueous solution using walnut hull. 2009. 90(2): p. 721-729
- Phosphate-intercalated Ca–Fe-layered double hydroxides: crystal structure, bonding character, and release kinetics of phosphate. J. Solid State Chem.. 2011;184(1):171-176.
- [Google Scholar]
- Highly efficient adsorption of Congo red in single and binary water with cationic dyes by reduced graphene oxide decorated NH2-MIL-68 (Al) J. Mol. Liq.. 2017;247:215-229.
- [Google Scholar]
- Xie, J., T. Yamaguchi, and J.-M.J.J.o.S.S.C. Oh, Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. 2021. 293: p. 121758.
- Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem.. 2021;293:121758
- [Google Scholar]
- Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI) Chem. Eng. J.. 2017;313:475-486.
- [Google Scholar]
- Effect of pH, ionic strength and temperature on sorption of Pb (II) on NKF-6 zeolite studied by batch technique. Chem. Eng. J.. 2011;168(1):86-93.
- [Google Scholar]
- Dual function magnetic hydroxyapatite nanopowder for removal of malachite green and Congo red from aqueous solution. Powder Technol.. 2016;302:207-214.
- [Google Scholar]
- Coal fly ash/CoFe 2 O 4 composites: a magnetic adsorbent for the removal of malachite green from aqueous solution. RSC Adv.. 2016;6(96):93564-93574.
- [Google Scholar]
- Super adsorption capability of rhombic dodecahedral Ca-Al layered double oxides for Congo red removal. J. Alloy. Compd.. 2018;768:572-581.
- [Google Scholar]
- Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater.. 2009;167(1–3):141-147.
- [Google Scholar]
- Fabrication of hierarchical bristle-grass-like NH4Al (OH) 2CO3@ Ni (OH) 2 core-shell structure and its enhanced Congo red adsorption performance. J. Alloy. Compd.. 2018;750:644-654.
- [Google Scholar]
- 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr (VI) ions. J. Hazard. Mater.. 2019;369:214-225.
- [Google Scholar]
- Highly efficient and rapid purification of organic dye wastewater using lignin-derived hierarchical porous carbon. J. Colloid Interface Sci.. 2022;625:158-168.
- [Google Scholar]
- Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci.. 2017;143:279-292.
- [Google Scholar]
- Zulfikar, M.A. and H.J.I.J.o.C.R. Setiyanto, Adsorption of congo red from aqueous solution using powdered eggshell. 2013. 5(4): p. 1532-1540.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104171.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2
Supplementary data 3
Supplementary data 3
Supplementary data 4
Supplementary data 4
Supplementary data 5
Supplementary data 5
Supplementary data 6
Supplementary data 6







