Translate this page into:
Removal of butyl xanthate and Cr(Ⅵ) using FeNi-biochar
* Corresponding author: E-mail address: zyy20172024@yeah.net (K. Bi)
-
Received: ,
Accepted: ,
Abstract
The magnetic FeNi-biochar (FeNi-BC) is prepared for butyl xanthate (BX) and Cr(Ⅵ) wastewater purification, which occurs via the adsorption and activation peroxodisulfate (PDS) degradation. The physical and chemical properties of FeNi-BC are analyzed by characterization technology, which is loaded on an FeNi alloy. According to Langmuir model calculation, the BX and Cr(Ⅵ) adsorption capacities of FeNi-BC are 366.21 and 29.56 mg/g, respectively. Adsorption mechanism analysis demonstrates that the functional groups on the surface of FeNi-BC contribute to BX and Cr(Ⅵ) removal. FeNi-BC can also be used for activating peroxysulphate (PDS) to remove BX and Cr(Ⅵ) with 100% and 97% removal, respectively. According to degradation mechanism analysis, the generated reactive oxygen species, such as SO4•– and 1O2 can transform BX into inorganic molecules. Cr(Ⅵ) is reduced to Cr(Ⅲ) by Fe(III)/Fe(II) and Ni(III)/Ni(II) cycles based on electron donors. The formation of FeNi on FeNi-BC changes the electronic structure, improving electron transfer ability and achieving excellent PDS activation performance for FeNi-BC.
Keywords
Adsorption
Butyl xanthate
Cr(Ⅵ)
FeNi-BC
Peroxysulphate
1. Introduction
As a country with numerous mining operations, China annually discharges approximately 200 million tons of mining wastewater [1]. Butyl xanthate (BX), a common flotation reagent, is malodorous and moderately toxic [2]. The direct discharge of mining wastewater containing BX would deteriorate water quality, posing a severe threat to the ecosystem and human health [3]. Ultimately, it impairs liver and kidney functions as well as the hematopoietic systems of both humans and animals [4]. Owing to the poor, complex, and fine ore resources in China, BX consumption has been increasing annually, leading to increasingly severe environmental pollution [5]. Cr(VI) is a toxic and dangerous heavy metal, which also exists in mining wastewater [6,7]. If mining wastewater containing BX or Cr(VI) is directly discharged into rivers and lakes without pretreatment, it can cause extreme harm to the ecological environment [8,9]. Hence, effectively solving this pollution issue is critical.
Recently, advanced oxidation processes (AOPs) and adsorption have been widely utilized for the efficient elimination of organic contaminants and heavy metals [10]. Reactive oxygen species generated from activating oxidants completely mineralize contaminants [11]. The persulfate-based advanced oxidation process is one of the AOPs [12]. The persulfate-based advanced oxidation process is the activation of peroxymonosulfate (PMS) or peroxydisulphate (PDS) through a physical or chemical activation method under external conditions by energy and electron transfer pathways [13]. This process generates SO4•– and HO• radicals by breaking the O-O chemical bond of PMS or PDS [14]. The SO4•– and HO• radicals can attack the pollutants and degrade them [15]. Besides, it can also achieve degradation by singlet oxygen (1O2) or non-radical pathways [16]. Compared with PMS, the molecular structure of PDS is symmetrical, with strong chemical stability in the reaction system [17]. Additionally, S2O82– has greater redox potential than HSO5–. Therefore, PDS is widely used in wastewater treatment [18]. However, the PDS-based advanced oxidation process requires a catalyst for generating reactive oxygen species (ROSs) owing to the weak oxidizing ability of PDS [19]. The catalyst should have excellent adsorption performance to enable the removal of pollutants. Therefore, it is necessary to prepare a catalyst that can effectively activate PDS for BX and Cr(VI) removal.
Biochar (BC) is a product of biomass pyrolysis. BC possesses an abundantly porous carbon structure and large specific surface area. Besides, BC has rich oxygen-containing functional groups and carbon defect sites [20]. Owing to its excellent adsorption capacity and redox activity for storing and transferring electrons, BC has garnered extensive attention in the environmental field [21]. Besides, the carbon defect structure and surface functional groups (such as C=O, -COOH, etc.) of BC can provide the activated active sites needed for activating PDS [22,23]. This reaction can break the O-O bond in PDS to produce ROSs that can attack and degrade the pollutants [24]. The surface functional groups of BC can adsorb and then reduce Cr(VI). However, the activation PDS performance and adsorption performance of BC are poor, which can be improved by the modified method.
Modification methods improving BC’s activation performance towards PDS have become a focus of research [25]. High-temperature carbonization is beneficial for producing more surface defects, promoting electron transfer, and generating more ROSs [26]. However, the high-temperature condition requires high energy consumption, which is not favorable for actual application [27]. The metal ions (such as Fe, Co, Ni, etc.) inherently have the property of activating PDS [28,29]. However, this process has the problem of low utilization of metal ions during activation PDS process [30]. Moreover, metal ions are prone to causing secondary pollution. To solve this limitation, researchers have tried to modify BC using metal compounds. Loading or doping metal ions onto BC can effectively overcome this problem [31]. Meanwhile, the addition of metal ions can modify the structure of BC and increase active sites to activate PDS [32,33]. Metal modification enables BC to possess magnetism, facilitating the separation of BC after use. Besides, the addition of iron-compounds can cause the reduction of Cr(VI), realizing Cr(VI) removal. This modification process also improves the BX and Cr(VI) adsorption performance of BC by increasing specific surface area and surface functional groups [32].
The multi-metal-modified BCs are conspicuously superior to the single-metal. Since several metals exist in synergy, it encompasses electron transfer and circulation among metals, contributing to the activation of PDS. Therefore, the multi-metals can synergistically enhance the activation performance of PDS. In this study, orange peels were used to prepare magnetic FeNi-BC, using Fe(NO3)3, ZnCl2, and Ni(NO3)3 as modification agents for BX and Cr(VI) removal. ZnCl2 was used as the chemical agent to improve BC’s pore structure. FeNi alloy is generated on BC by the decomposition of Fe(NO3)3 and Ni(NO3)3, which is used to activate PDS.
2. Materials and Methods
2.1. Material
The orange peels were obtained from the local market. Zinc chloride was purchased from Tianjin Hongyan Reagent Co., Ltd. Ferric nitrate, nickel nitrate, and citric acid were purchased from Tianjin Comeo Chemical Reagents Co, Ltd. The K2Cr2O7 was ordered from Aladdin Chemistry Reagent Co. Ltd.
2.2. Preparation FeNi-BC
The orange peel (12 g) was mixed with 18 g of ZnCl2, 6 g of Fe(NO3)3, and 4 g of Ni(NO3)2 in 150 mL of distilled water. Then, the mixture was dried in the electric oven at 80°C for 12 h. The dried sample (10 g) was placed in the resistance furnace and heated at 900°C for 60 mins in an N2 atmosphere with an N2 flow rate of 150 mL/min [34]. After heating, the residue in the resistance furnace was FeNi-BC. The characterization of the sample has been detailed in the supplementary material.
2.3. Adsorption and activation PDS degradation experiment
The method of the adsorption experiment and activation PDS degradation experiment has been detailed in the supplementary material.
3. Results and Discussion
3.1. Characterization of FeNi-BC
Figure 1(a) shows the X-ray Diffraction (XRD) analysis of FeNi-BC. FeNi-BC has several characteristic peaks of FeNi with high crystallinity, indicating that FeNi is generated on FeNi-BC by Ni(NO3)3 and Fe(NO3)3 decomposition (JCPDS PDF#47-1405). FeNi can effectively activate the PDS to produce the SO4–·. The SO4–· can also react with hydroxyl ions in solution to form HO·, which can oxidize most organic matter and even some inorganic matter, achieving pollutant removal. The existence of the FeNi alloy enables FeNi-BC to exhibit magnetism, contributing to BX and Cr(VI) removal under the condition of an applied magnetic field [35].
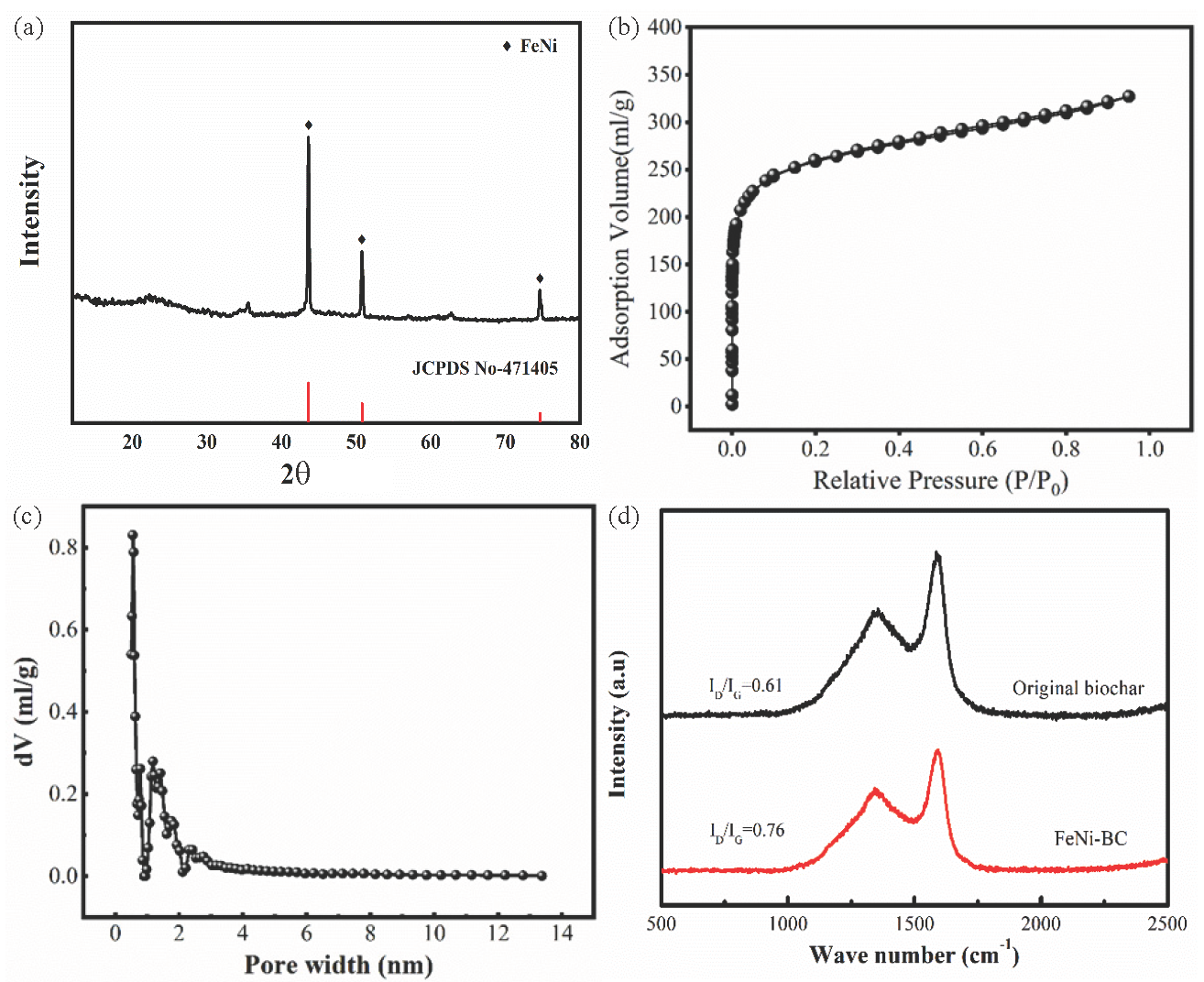
- (a) XRD analysis of, (b-c) nitrogen adsorption curve and pore size distribution, and (d) Raman spectra analysis of the FeNi-BC.
Figure 1(b-c) depict the nitrogen adsorption curve and pore size distribution of FeNi-BC. As shown in Figure 1(b), when P/P0 is less than 0.1, the nitrogen adsorption capacity of FeNi-BC rapidly increases, indicating that FeNi-BC exists in the micropores. As the P/P0 ratio continuously increases, the nitrogen adsorption curve continues to increase, with the surface area of 962.40 m2/g. As Figure 1(c) displays, it can be concluded that FeNi-BC has a large nitrogen adsorption capacity when the pore size of FeNi-BC is around 1 nm.
Figure 1(d) shows the Raman spectra analysis of the FeNi-BC and original BC. The disordered (Csp3) and graphitic (Csp2) structures appear at around 1350 and 1600 cm–1, corresponding to the D and G bands, respectively. The ID/IG ratio of the FeNi-BC was 0.76, which is larger than that of the original biochar (0.61). This result proves that FeNi-BC has a large disordered degree after modification, indicating the formation of a disordered structure in FeNi-BC [36]. This result also demonstrated that FeNi-BC forms a defect structure, which contributes to BX and Cr(Ⅵ) removal in the FeNi-BC/PDS system.
Figure 2(a-c) show the scanning electron microscope (SEM) micrographs and energy dispersive spectroscopy (EDS) images of FeNi-BC. As Figure 2(a-b) shown, the FeNi-BC exhibits a developed pore structure, which is consistent with the nitrogen adsorption curve analysis. This result also indicated that FeNi-BC can provide a significant number of adsorption sites for BX and Cr(Ⅵ) removal. FeNi-BC exhibited grayish white particles, which were analyzed using an EDS image. As shown in Figure 2(c), FeNi-BC has Ni and Fe elements. It was concluded that the grayish white particle was FeNi combined with XRD analysis.
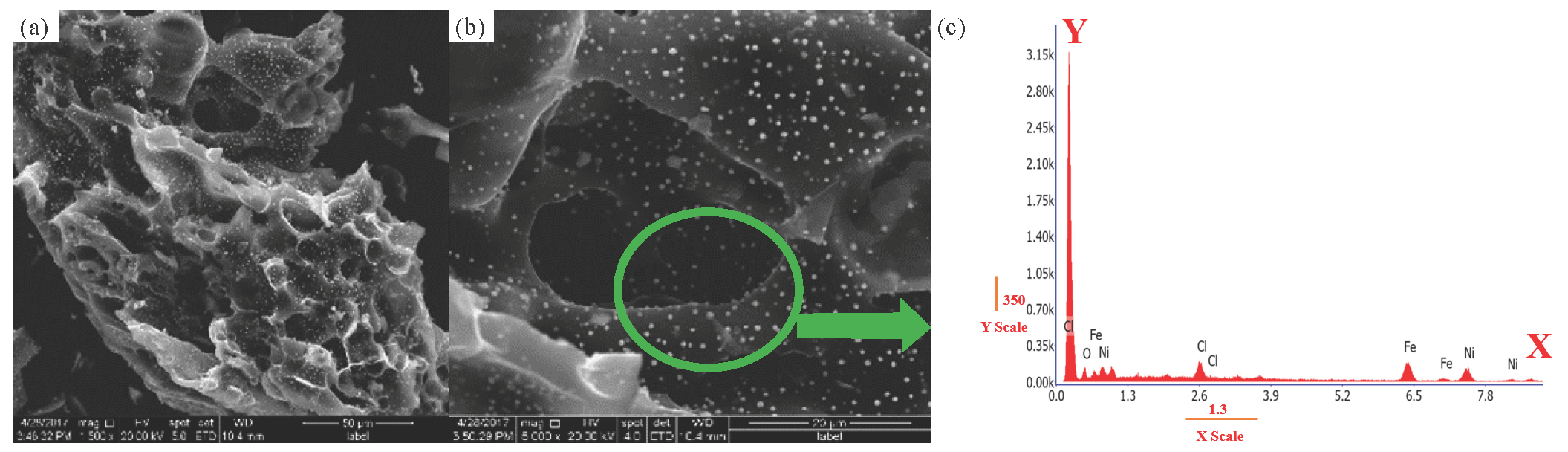
- (a-b) SEM images and (c) EDS images of FeNi-BC. The green circle delineates the specific range of the energy spectrum under consideration. The result is in the figure 2c.
3.2. Adsorption BX and Cr(Ⅵ)
3.2.1. Comparison of BX and Cr(Ⅵ) adsorption amount of FeNi-BC and original biochar
The BX and Cr(Ⅵ) adsorption amount of the FeNi-BC and original biochar (BC) have been shown in Figure S1. As Figure S1 shows, the BX and Cr(Ⅵ) adsorption amount of FeNi-BC after modification increased compared to BC. The reason is that FeNi-BC exhibits a developed pore structure and rich defect structure, contributing to BX and Cr(Ⅵ) removal.
3.2.2. Influence of adsorption parameter
3.2.2.1. Influence of pH
The effect of initial pH on BX and Cr(Ⅵ) adsorption was analyzed (Figure 3). As Figure 3(a) shows, the BX adsorption amount generally decreased at pH=2-7. It can be explained that electrostatic repulsion occurs between BX and FeNi-BC as the pH increases due to the presence of large amounts of OH– in the aqueous solution. BX is a kind of anionic organic compound. Therefore, the OH– competes with C4H9OCSS–, which hinders the interaction between C4H9OCSS– and FeNi-BC, resulting in low BX adsorption capacity. The electrostatic repulsion between C4H9OCSS– and FeNi-BC was weak at a low pH value. Therefore, FeNi-BC exhibited large BX adsorption capacity at pH 2. The zero potential charge of FeNi-BC was about pHpzc=4.2 (Figure S2). The surface of the FeNi-BC was positively charged at pH <4.2, which is favorable for BX adsorption [37]. The FeNi-BC potential was negatively charged at pH >4.2. Therefore, BX adsorption amount generally decreases at pH > 4.2.
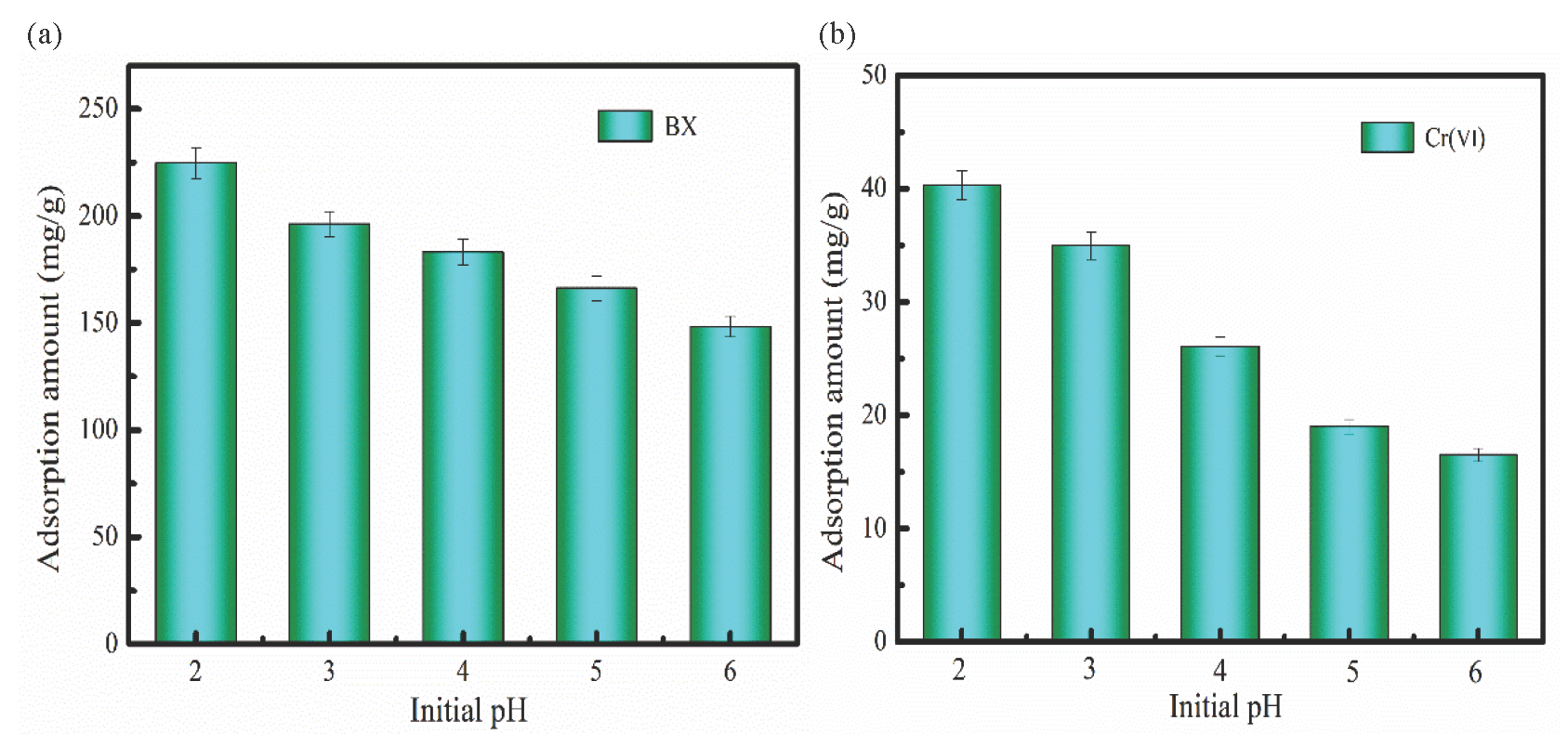
- The influence of the initial pH on (a) BX and (b) Cr(Ⅵ) adsorption (BX and Cr(VI)concentration: 200 mg/L, FeNi-BC dosage:0.1g).
Cr(VI) exists in several forms at different pH values. At a pH value between 3-7, Cr(VI) can form the HCrO4– or Cr2O72–. While Cr(VI) is in the form of the CrO42– at pH > 6.8. Figure 3(b) shows that FeNi-BC exhibits large Cr(VI) adsorption capacity at low pH. The aqueous solution has a huge amount of hydrogen ions, which make FeNi-BC have a positive charge. Thus, Cr(VI) (HCrO4– or Cr2O72–) can be adsorbed on FeNi-BC owe to electrostatic adsorption. The potential of the FeNi-BC is negatively charged at pH>4.2. With an increase in pH value, hydrogen ion content generally decreases. Therefore, Cr(VI) adsorption amount generally decreases at pH =3-6 owing to electrostatic repulsion.
3.2.2.2. Influence of adsorption time
The adsorption rate is an important indicator that reflects the adsorption performance of adsorbent. While adsorption capacity is directly related to the contact time between the adsorbent and pollutant. As shown in Figure 4(a), with increasing in adsorption time, the BX and Cr(VI) adsorption amount are general increase. BX adsorption on FeNi-BC is taken as an example to analyze the adsorption process. As Figure 4(a) shown, BX adsorption on FeNi-BC is in a rapid growth period from 0 to 100 mins. The reason is that FeNi-BC has many adsorption sites, and the adsorption solution contains a high BX concentration, resulting in a fast adsorption rate. The BX adsorption on FeNi-BC is in a slow growth period from 160 to 400 mins. The reason is that most of the adsorption sites are occupied. Besides, BX concentration in the solution is low compared to the initial adsorption stage. After adsorption for 400 mins, BX adsorption on FeNi-BC reaches equilibrium.
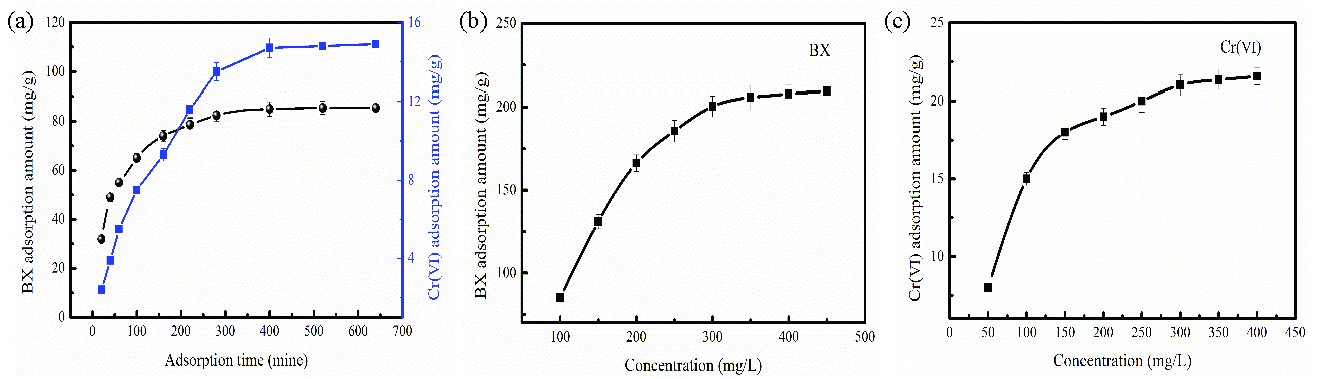
- (a) Influence of adsorption time, (b-c) initial concentration (a=BX and Cr(VI) concentration: 100 mg/L, b-c= adsorption time: 640 mins, FeNi-BC dosage: 0.1g, pH:5).
3.2.2.3. Influence of initial concentration
The pollution concentration in wastewater is also an important indicator for estimating pollution removal in the actual application process. As Figures 4(b-c) show, the BX and Cr(VI) adsorption amount gradually increased with the increase in initial concentration. It can be explained that the BX and Cr(VI) amount adsorbed per unit mass increased as initial BX and Cr(VI) concentration increased. It achieved a high driving force, which increased the collision probability between the sorbent and adsorbent [38]. Cr(VI) adsorption was taken as an example to analyze the influence of initial concentration on the adsorption process. When Cr(VI) concentration increases from 300 to 400 mg/L, the increase of Cr(VI) adsorption amount is slow owe to lack of adsorption sits. This result also indicates that the adsorption binding sites gradually reach saturation.
3.2.2.4. Influence of impurity ions
The actual wastewater usually contains a considerable amount of coexisting ions. These coexisting ions can influence the adsorption performance of FeNi-BC for BX and Cr(VI). The influence of SO42–, Cl–, Cd2+, and Pb2+ on BX and Cr(VI) adsorption was investigated. As shown in Figure S3, the coexisting ions hardly hinder BX and Cr(VI) adsorption with little decrease in removal. The strong interaction of hard oxygen atoms of FeNi-BC with the hard Cr(VI) cations shows high selectivity. The soft Pb(II) and Cd(II) cations have low interfering effect, based on the Hard-Soft interaction theory [39]. It is also hypothesized that coexisting ions might impede the combination of FeNi-BC with C4H9OCSS– and HCrO4–/Cr2O72–, decreasing the probability of effective collisions between FeNi-BC and C4H9OCSS–/HCrO4–/Cr2O72–. Besides, the coexisting ions can bind with the adsorption sites of FeNi-BC occupying those originally belonging to BX and Cr(VI). Thus, BX and Cr(VI) removal is only slightly decreased.
3.2.3. Adsorption kinetics study
Adsorption kinetics mainly investigate the rate of adsorption of the pollutant. The study of adsorption kinetics can explore the factors influencing the adsorption rate and the possible mechanisms in the process [40].
BX and Cr(VI) adsorption processes are analyzed using Pseudo-first/second order and Intraparticle diffusion models [41]. Table 1 presents fitting results of the three kinds of the adsorption kinetic models. As Table 1 shows, the correlation coefficient R2 of the pseudo-second order models was greater than that of the pseudo-first order and intraparticle diffusion models. Consequently, the BX and Cr(VI) adsorption processes complied with the pseudo-second-order kinetic models. The qe,cal values of the BX and Cr(VI) are close to experimental values, which also indicates that the adsorption kinetics process of the BX and Cr(VI) can be analyzed by the pseudo-second order model. BX and Cr(VI) adsorption data fitting the pseudo-second-order equation have been shown in Figure 5(a-b).
| Kinetics models | Models parameter | Fitting result | |
|---|---|---|---|
| BX | Cr(VI) | ||
| Pseudo-first order | qe.cal (mg/g) | 80.37 | 14.71 |
| K1 (1/min) | 0.0225 | 0.0077 | |
| R2 | 0.9617 | 0.9486 | |
| qe.cal (mg/L) | 91.16 | 18.83 | |
| Pseudo-second order | K2 (g/mg min) | 0.3956 | 7.55 |
| R2 | 0.9989 | 0.9909 | |
| C | 25.26 | 27.20 | |
| Intraparticle diffusion | K32 (mg/g min1/2) | 3.034 | 3.2707 |
| R2 | 0.8410 | 0.9628 | |
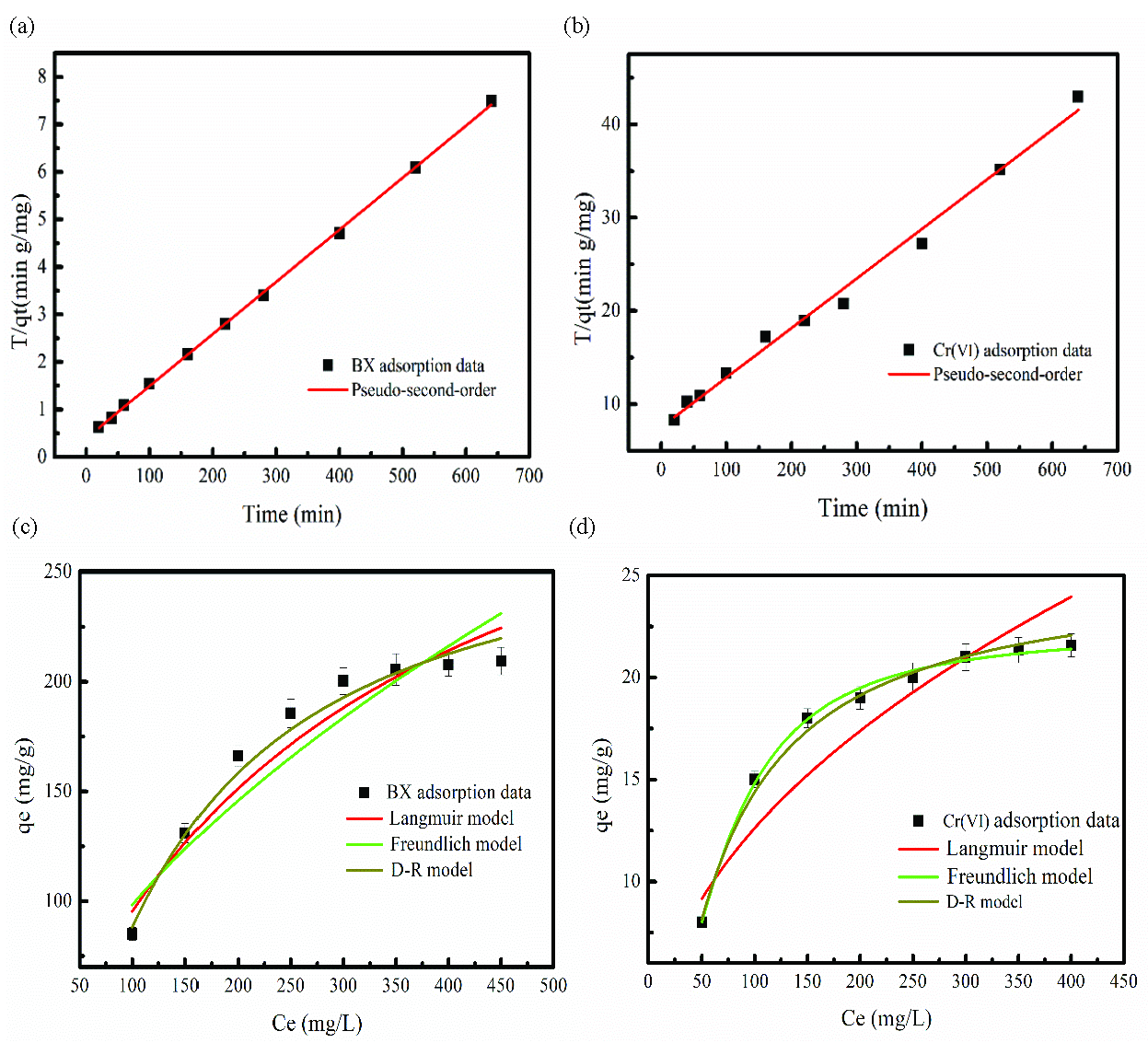
- BX and Cr(VI) adsorption data fitting the (a-b) Pseudo-second order, and (c-d) adsorption isotherm model (a-b=BX and Cr(VI) concentration:100 mg/L, b-c= adsorption time: 8 h, FeNi-BC dosage: 0.1 g, pH: 5).
The adsorption process usually consists of four steps: bulk diffusion process, film diffusion process, interarticular diffusion process, and solute sorption process [42]. To further investigate the BX and Cr(VI) adsorption process, the BX and Cr(VI) adsorption data are fitted using the intraparticle diffusion model. As shown in Figure S4, the entire adsorption process was divided into three parts. The 0-60-min period was a rapid adsorption process, characterized by the film diffusion stage. The BX and Cr(VI) mainly combined with active groups on the outer surface of FeNi-BC. The adsorption rate decreased from 100 to 280 mins. At the moment, the BX and Cr(VI) entered the interior of the FeNi-BC and combined with the groups in the pores of the FeNi-BC. The interarticular diffusion was dominant in this period. After 400 mins, the adsorption rate and desorption rate were almost the same, indicating that BX and Cr(VI) adsorption on FeNi-BC reach adsorption equilibrium. Since the entire BX and Cr(VI) adsorption process was not a straight line passing through the origin (Figure S4). It can be guessed that both the film diffusion process and the interarticular diffusion process simultaneously exist during the BX and Cr(VI) adsorption process.
3.2.4. Adsorption isotherms study
BX and Cr(VI) adsorption on FeNi-BC are investigated by Langmuir, Freundlich, and Dubinin-Radushkevish (D-R) models [43]. The correlation between the adsorption isotherm model and BX/Cr(VI) adsorption data were analyzed to predict the adsorption capacity of FeNi-BC for BX and Cr(VI). Figure 5(c-d) show that the BX and Cr(VI) adsorption data fit the adsorption isotherm model. Under different initial concentrations, the adsorption amounts of BX and Cr(VI) both increase as the initial concentration increases. The main reason is that the concentration difference between the surface of the FeNi-BC and the adsorption solution becomes large as the initial concentration increases. However, with an increase in the BX and Cr(VI) adsorption amount, the adsorption sites of the FeNi-BC becomes limited until adsorption saturation. The fitting result has been presented in Table 2. As Table 2 shows, the R2 values of the Langmuir and D-R model were larger than that of the Freundlich model for BX and Cr(VI) adsorption on FeNi-BC. This result proves that BX and Cr(VI) adsorption process can be well explained by the Langmuir model, confirming the monolayer adsorption of BX and Cr(VI) on FeNi-BC. According to Langmuir analysis, the adsorption amounts of BX and Cr(VI) were 366.21 and 29.56 mg/g, respectively. The E values of the BX and Cr(VI) calculated from the D-R model were 14.26 and 12.94 kJ/mol, respectively. Furthermore, the E value is about 8-16 kJ/mol in the ion exchange or chemical reactions [44,45].
| Isotherm models | Models parameter | Fitting result | |
|---|---|---|---|
| BX | Cr(VI) | ||
| Freundlich | R2 | 0.9044 | 0.8963 |
| n/1 | 0.5679 | 0.4623 | |
| KF ((mg/g).(L/mg)1/n) | 7.1988 | 1.5015 | |
| R2 | 0.9490 | 0.9656 | |
| qm (mg/g) | 366.21 | 29.56 | |
| Langmuir | KL (L/mg) | 0.00352 | 0.00835 |
| R2 | 0.9866 | 0.9954 | |
| D-R | QD-R | 285.65 | 25.46 |
| β | 0.0025 | 0.003 | |
| E(kJ/mol) | 14.26 | 12.94 | |
D-R: Dubinin-Radushkevich.
3.2.5. BX and Cr(Ⅵ) adsorption mechanism
To investigate the adsorption mechanism of BX and Cr(VI), FT-IR spectra of FeNi-BC before and after BX/Cr(VI) adsorption were analyzed. As Figure 6(a) shows, the -CH bending vibration appeared at 876 cm–1 [46]. However, the corresponding peak intensity of the -CH group changed after BX/Cr(VI) adsorption. This result proves that the π-π interaction contributes to BX/Cr(VI) adsorption. The stretching vibration of O-H at 3424 cm–1 is migrated to 3336 and 3340 cm–1 after BX/Cr(VI) adsorption, respectively. It can be explained by the formation of hydrogen bonding between NiFe-BC and BX/Cr(VI) in the adsorption process [47].
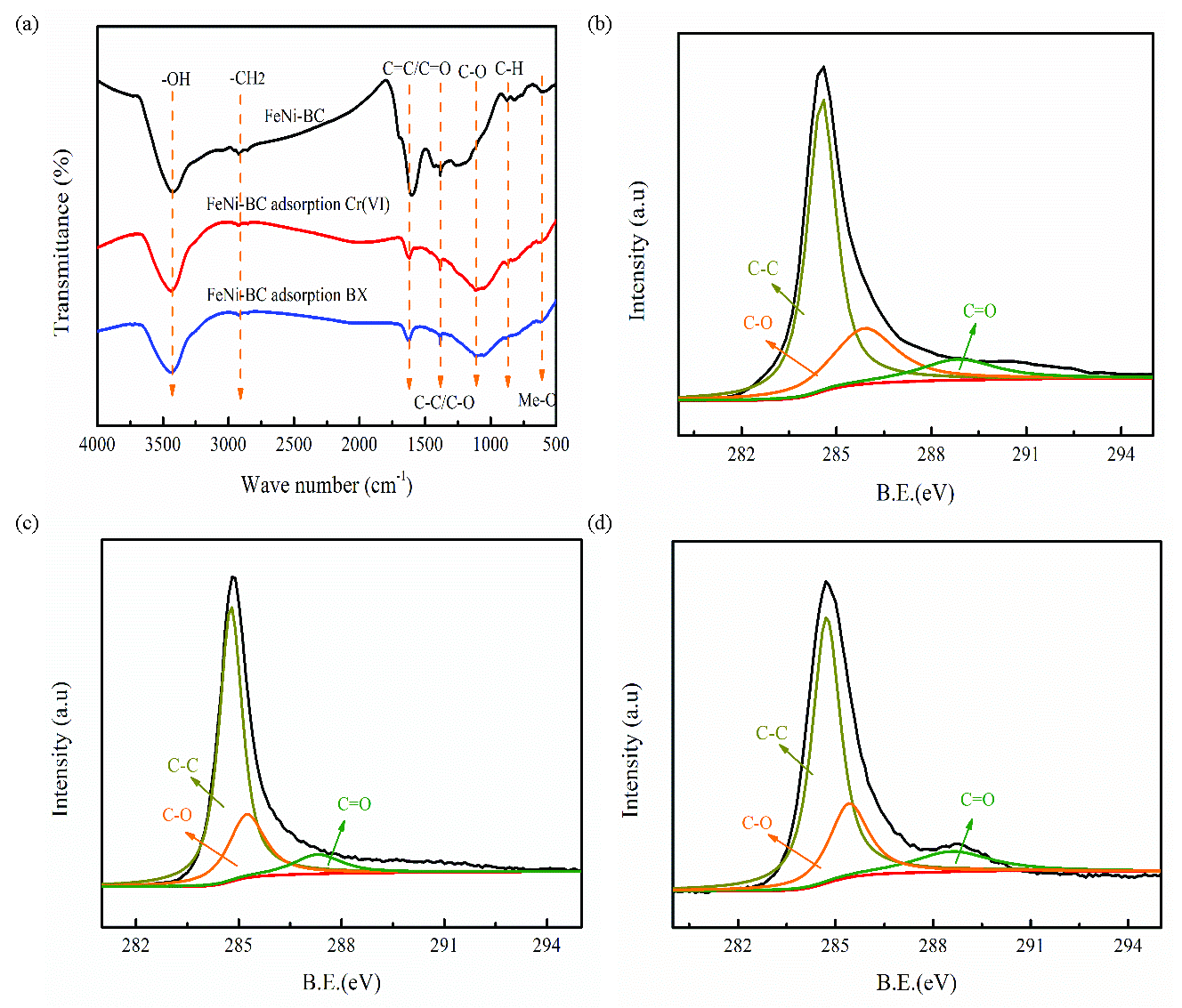
- (a-b) FT-IR spectra of FeNi-BC before and after BX/Cr(VI) adsorption, (c-d) C1s spectra analysis of NiFe-BC before and after BX/Cr(VI).
Figures 6(b-d) show C1s spectra analysis of NiFe-BC before and after BX/Cr(VI) adsorption. The C-O and C=O bond binding energies changed after BX adsorption. The C-O and C=O peak areas decreased by 2.08% and 2.11 % after BX adsorption, respectively. The peak area of the C-O group decreases from 26.07% to 24.06 % after Cr(VI) adsorption. However, the peak area of the C=O group increased by 1.24%. The reason is that the C-O group oxidized into the C=O group owing to the electron-donating group of the C-O band in the Cr(VI) adsorption process. Therefore, Cr(Ⅲ) is generated by the reduction reaction of the Cr(VI) [48].
3.2.6. Reusability of the FeNi-BC
The recyclability of BX/Cr(VI) adsorption on FeNi-BC was investigated after regeneration for actual application (Figure S5). As shown in Figure S5, with an increase in the cycle time, the BX/Cr(VI) adsorption amount generally decreased. The reason is that some adsorption sites of NiFe-BC were destroyed after regeneration. Besides, some BX/Cr(VI) binding with adsorption sites cannot be desorbed.
3.3. Activation PDS degradation BX and Cr(Ⅵ)
3.3.1. Effect of initial BX and Cr(Ⅵ) concentration
The BX and Cr(Ⅵ) degradation performances at different initial concentrations were analyzed (Figures 7a-b). Besides, BX and Cr(Ⅵ) degradation data were fitted using the first-order kinetic model, and the relevant kinetic parameters have been shown in Figures S6(a-b). As Figures 7(a-b) show, both degradation removal and apparent reaction rate of the BX and Cr(Ⅵ) gradually decrease with an increase in BX and Cr(Ⅵ) initial concentration. For instance, when the BX initial concentration is 60 mg/L, the degradation removal of BX reaches 100% after 120 mins. The BX is completely degraded with the apparent reaction rate of 0.0309 min–1. When the BX initial concentration is 100 mg/L, the degradation removal is 78%. BX degradation removal decreased by 22% compared to BX initial concentration of 60 mg/L. PDS needs to be adsorbed on the surface of FeNi-BC for activation and subsequently generates ROSs during the degradation process. When the BX initial concentration was relatively high, an excessive amount of BX was adsorbed on FeNi-BC, occupying limited active sites of FeNi-BC. Competitive adsorption occured. Therefore, the generation process of the ROSs is suppressed. Besides, with increase in the BX concentration, the number of BX molecules in the unit reaction solution increases. The limited activators FeNi-BC and PDS could only provide a certain amount of ROSs, which are insufficient to completely degrade a large amount of BX, resulting in a reduction in the BX degradation removal.
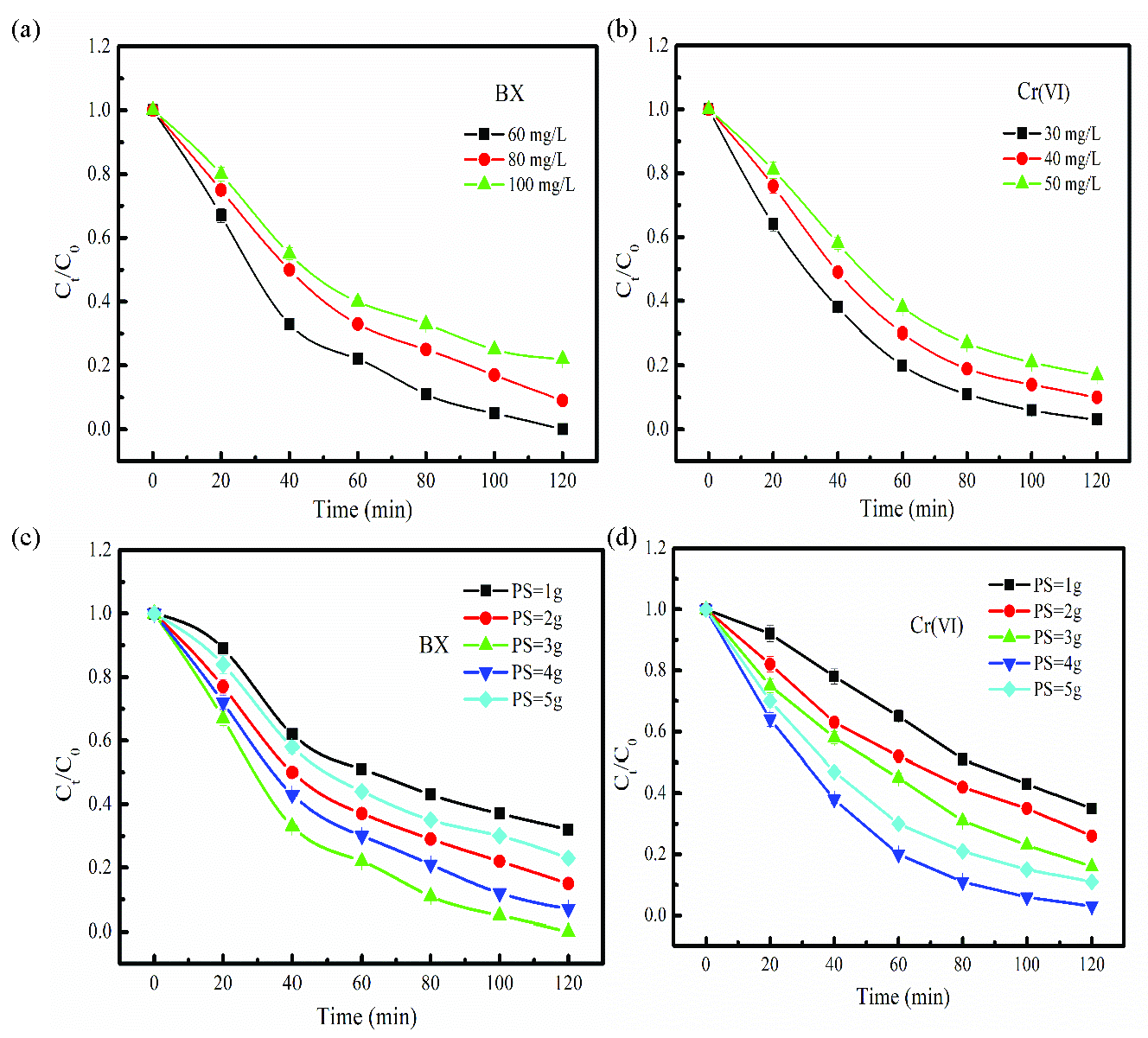
- The degradation performance of the different (a) BX and (b) Cr(VI), (c) concentration, different PDS dosage for BX and (d) Cr(VI) in the FeNi-BC/PDS degradation system. (a =PDS:3g, b=PDS 4g:L, c= BX concentration: 60 mg/L, d=Cr(Ⅵ) concentration: 30 mg/L, FeNi-BC dosage: 0.1g).
3.3.2. Effect of PDS dosage
PDS is a precursor for generation of the ROSs, and PDS dosage is a key influencing factor in the BX and Cr(Ⅵ) degradation system. The BX and Cr(Ⅵ) degradation removal within the range of PDS dosage from 1 g to 5g have been shown in Figure 7(c-d). Besides, BX and Cr(Ⅵ) degradation data are fitting using first-order kinetic model, and the related kinetic parameters are shown in Figure S6(c-d). As shown in Figure 7(c-d), BX and Cr(Ⅵ) degradation removal significantly increased from 68% to 100 % and 65% to 97% as PS dosage increased from 1g to 3g and 1g to 4g, respectively. Besides, the apparent reaction rate of the BX and Cr(Ⅵ), the degradation process also increases. The low PDS dosage exerts a certain inhibitory effect on the BX and Cr(Ⅵ) degradation process. Subsequently, the generated ROSs are not adequate for BX and Cr(Ⅵ) degradation in the FeNi-BC/PDS degradation system, resulting in a relatively low degradation removal. Under high PDS dosage, the contact probability of PDS and FeNi-BC per unit time increases, resulting in the generation of more ROSs. PDS, as an electron acceptor, collaborates with the FeNi-BC to facilitate the degradation of BX and Cr(Ⅵ). Therefore, BX and Cr(Ⅵ) degradation removal are improved. However, when the PDS dosage increased from 3g to 5g, and 4g to 5g, the degradation removal of BX and Cr(Ⅵ) gradually decreased from 100 % to 73% and 97% to 89%, respectively. The excess PDS was demonstrated to induce ROSs quenching reactions [49]. Besides, excessive PDS dosage could inhibit BX and Cr(Ⅵ) adsorption on FeNi-BC, probably due to the competitive adsorption between BX/Cr(Ⅵ) and PDS [50].
3.3.3. Effect of impurity ion
The composition of actual wastewater is complex, containing large quantities of inorganic cations and anions. To investigate the applicability of the FeNi-BC/PDS degradation system in natural environmental conditions, common inorganic cations and anions that may exist in actual wastewater are introduced to investigate their interference with the FeNi-BC/PDS degradation system. The influence of SO42–, Cl–, Cd2+, and Pb2+ on BX and Cr(VI) degradation and removal was explored (Figure S7). The analysis results showed that the addition of SO42–, Cl–, Cd2+, and Pb2+ has no obvious influence on the final degradation removal of BX and Cr(VI). In conclusion, the inorganic ions introduced in the FeNi-BC/PDS system has little influence on the BX and Cr(VI) degradation process.
3.3.4. Activation PDS degradation mechanism analysis
The SO4•– and •OH were the two predominant ROSs in the activation of the PDS degradation system. PDS can be activated at the active sites to produce O⋅2–. Besides, singlet oxygen (1O2), a non-radical active species, is also regarded as an effective ROS for oxidizing pollution. 1O2 has high selectivity, which is less influenced by the environment. Besides, it can oxidize the pollutants that resist other active species. The methanol (MeOH), tert-butanol (TBA), p-benzoquinone (BQ), and furfuryl alcohol (FFA) were added into the FeNi-BC/PDS system to determine which free radical or non-radical 1O2 played the major role in the BX degradation process using the free radical quenching method [51]. As shown in Figure S8, when no quenching agent was added, the FeNi-BC/PDS system exhibited an excellent BX degradation removal. The BX degradation process was inhibited when MeOH, TBA, and BQ were added to the FeNi-BC/PDS system. However, the FeNi-BC/PDS degradation system still showed large BX degradation removal, indicating that it produces the SO4•–, •OH, and O2.–. FeNi-BC/PDS degradation system has low BX degradation removal after adding the FFA compared to the addition of MeOH, TBA and BQ. The result of the quenching experiment also confirms that 1O2 is indeed generated in the FeNi-BC/PDS degradation system, which plays an important role in the BX degradation process. Based on the above analysis, it can be inferred that PDS is catalyzed by FeNi-BC to generate 1O2, SO4•–, •OH, and O2 in the FeNi-BC/PDS degradation system Eqs. (1-6). Besides, the surface functional groups of the FeNi-BC can also activate PDS to generate ROSs. Finally, BX is transformed into non-toxic CO2 and H2O Eq. (7-9). The BX degradation process is described in the following equations.
FeNi-BC activation PDS reaction contributes to the generation of Fe(II) (Eq. 1). The Ni(II) is generated based on the Eq. (4), (5) [52]. Subsequently, FeNi-BC/PDS system has high Fe(II) and Ni(II) concentration. Moreover, the Fe(III) and Ni (III) concentration increases due to the reaction between Fe(II)/ Ni(II) and PDS (Eq. 6). In the FeNi-BC/PDS-Cr(VI) system, the content of Fe(II) is low compared to the FeNi-BC/PDS-BX system. The Ni(II) content also decreases in the FeNi-BC/PDS-Cr(VI) system compared to the FeNi-BC/PDS-BX system. These results indicated that Fe(II) and Ni(II) are involved in reduction reaction of the Cr(VI) [53]. The mechanism scheme for BX and Cr(VI) degradation in the FeNi-BC/PDS system has been shown in Figure 8.
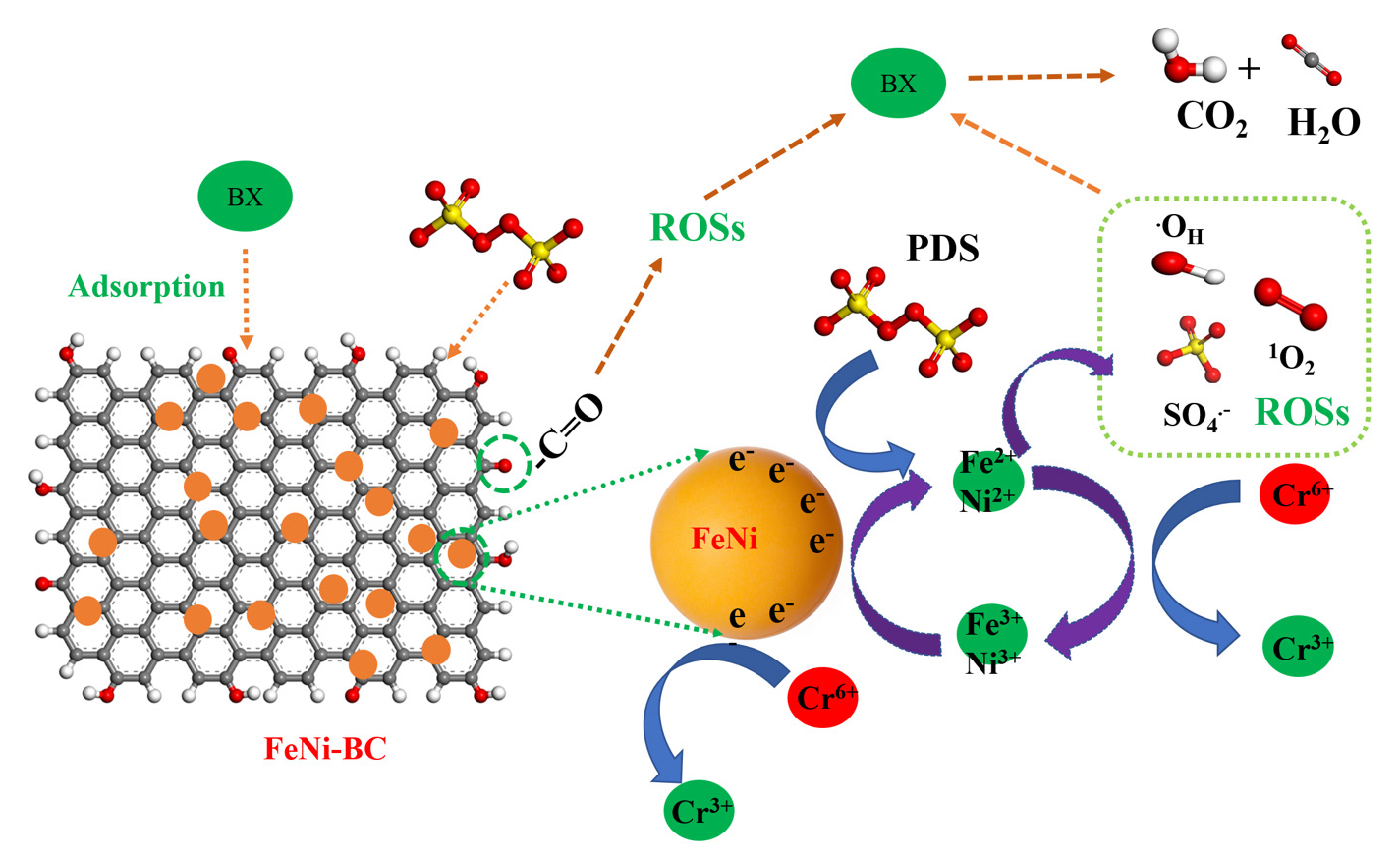
- (a) The free radical quenching for BX degradation and mechanism scheme for BX and Cr(VI) degradation in FeNi-BC/PDS degradation system.
3.3.5. Reusability of the FeNi-BC/PDS system
To comprehensively assess the practical application prospects of the FeNi-BC/PDS degradation system, it is of great significance to investigate the reusability of the FeNi-BC/PDS system. This work performs five cycles of the reusability degradation experiment, with the first experiment using freshly prepared FeNi-BC. The subsequent four cycles of experiments use the solid-liquid separated FeNi-BC after degradation. The results of the BX and Cr(VI) degradation experiments over multiple cycles have been shown in Figure S9. It was observed that the degradation removal of BX and Cr(VI) gradually decreased as the cycles increased. The reason might be that the activity of the FeNi-BC/PDS system declines as cycles increase. Besides, the residual BX/Cr(VI) and the generated intermediate products occupy the active sites, reducing the activity of the FeNi-BC. After five cycles, the degradation removal of the BX and Cr(VI) can still reach 81.95% and 78.26%, respectively.
4. Conclusions
The FeNi-BC is prepared for BX and Cr(Ⅵ) removal by adsorption and activation PMS degradation. The BX and Cr(Ⅵ) adsorption process are influenced by the adsorption parameters. BX and Cr(Ⅵ) adsorption on FeNi-BC are chemisorption, based on Pseudo-second order model analysis. The surface functional groups and π-π interaction of the FeNi-BC contribute to BX and Cr(Ⅵ) removal. FeNi-BC activation PDS achieves efficient removal of the BX and Cr(Ⅵ) with removal of 100% and 97%, respectively. The generated ROSs such as SO4•– and 1O2 play an important role in the BX degradation process. The Fe(III)/Fe(II) and Ni(III)/Ni(II) cycling contribute to reduction of the Cr(Ⅵ). The above work demonstrates that the FeNi-BC achieves BX and Cr(Ⅵ) removal, providing a novel route of waste-to-waste remediation.
Acknowledgment
The authors would like to express their gratitude to the Specialized Research Fund for Henan Province Science and Technology Research Project (252102320019) for financial support.
CRediT authorship contribution statement
Shuqi Feng: Methodology, Formal analysis, Writing - original draft. Kejun Bi: Methodology, Resources, Supervision, Writing-original draft. Tianlun Cui: Methodology, Validation. Shanshan Zhao: Investigation, Resources, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary data
Supplementary material to this article can be found online at https://dx.doi.org/10.25259/AJC_55_2025.
References
- A new strategy for colorimetric detection and removal of butyl xanthate in mineral processing wastewater: Based on a novel nanozyme of Ag@Fe3O4-MnO2. Journal of Environmental Chemical Engineering. 2024;12:113391. https://doi.org/10.1016/j.jece.2024.113391
- [Google Scholar]
- A high-efficiency clay mineral based organic photocatalyst towards photodegradation of butyl xanthate in mineral processing wastewater. Separation and Purification Technology. 2024;349:127880. https://doi.org/10.1016/j.seppur.2024.127880
- [Google Scholar]
- Adsorption-catalytic synergistic fenton degradation of potassium butyl xanthate in flotation tailing wastewater by renewable iron-loaded sludge: Performance, kinetics and mechanism. Separation and Purification Technology. 2025;359:130533. https://doi.org/10.1016/j.seppur.2024.130533
- [Google Scholar]
- Enhancing photocatalytic degradation of potassium butyl xanthate in mineral processing wastewater: Novel VO2+-doped LiZnBO3 nanosheets under visible light. Surfaces and Interfaces. 2023;42:103459. https://doi.org/10.1016/j.surfin.2023.103459
- [Google Scholar]
- A newly-constructed bifunctional bacterial consortium for removing butyl xanthate and cadmium simultaneously from mineral processing wastewater: Experimental evaluation, degradation and biomineralization. Journal of Environmental Management. 2022;316:115304. https://doi.org/10.1016/j.jenvman.2022.115304
- [Google Scholar]
- Sustainable bifunctional ZnO composites for synchronous adsorption and reduction of Cr(VI) to Cr(III) Environmental Science and Pollution Research International. 2023;30:80545-80558. https://doi.org/10.1007/s11356-023-28169-6
- [Google Scholar]
- Modification of clinoptilolite nano-particles with hexadecylpyridynium bromide surfactant as an active component of Cr(VI) selective electrode. Journal of Industrial and Engineering Chemistry. 2013;19:2026-2033. https://doi.org/10.1016/j.jiec.2013.03.018
- [Google Scholar]
- Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights. Environmental Science and Pollution Research International. 2018;25:25629-25637. https://doi.org/10.1007/s11356-016-8342-1
- [Google Scholar]
- Pyrolysis of plastic waste: Opportunities and challenges. Journal of Analytical and Applied Pyrolysis. 2020;152:104804. https://doi.org/10.1016/j.jaap.2020.104804
- [Google Scholar]
- Electrochemical oxidation degradation of xanthate and its mechanism: Effects of carbon chain length and electrolyte type. Journal of Cleaner Production. 2024;448:141626. https://doi.org/10.1016/j.jclepro.2024.141626
- [Google Scholar]
- Enhanced activity of clinoptilolite-supported hybridized PbS–CdS semiconductors for the photocatalytic degradation of a mixture of tetracycline and cephalexin aqueous solution. Journal of Molecular Catalysis A: Chemical. 2015;408:152-160. https://doi.org/10.1016/j.molcata.2015.07.017
- [Google Scholar]
- Complexation between butyl xanthate and Cu enhanced peroxydisulfate activation and cation redox cycle by Fe-Cu-LDH/biochar. Journal of Environmental Chemical Engineering. 2024;12:112466. https://doi.org/10.1016/j.jece.2024.112466
- [Google Scholar]
- Electrochemical activation of peroxydisulfate by Ti/IrTaO2 anode for quick degradation of xanthate. Journal of Environmental Chemical Engineering. 2024;12:111740. https://doi.org/10.1016/j.jece.2023.111740
- [Google Scholar]
- 2D nanosheet CoOx/BiVO4 heterojunction for promoting peroxysulphate activation: Performance and mechanistic. Environmental Technology & Innovation. 2023;32:103337. https://doi.org/10.1016/j.eti.2023.103337
- [Google Scholar]
- Research progress in photocatalytic activated persulfate degradation of antibiotics by bismuth-based photocatalysts. Separation and Purification Technology. 2023;324:124628. https://doi.org/10.1016/j.seppur.2023.124628
- [Google Scholar]
- Thermal modified sediments photocatalyst: A new strategy using waste to treat waste for tetracycline degradation and transformation product-associated risks. Journal of Water Process Engineering. 2024;64:105719. https://doi.org/10.1016/j.jwpe.2024.105719
- [Google Scholar]
- Effects of excess sludge composting process, environmentally persistent free radicals, and microplastics on antibiotics degradation efficiency of aging biochar. Bioresource Technology. 2024;393:130070. https://doi.org/10.1016/j.biortech.2023.130070
- [Google Scholar]
- Reducing agents enhanced prometon degradation by CuBi2O4/peroxymonosulfate: Development of interfacial electron transport and circulation of Cu+/Cu2+. Chemical Engineering Journal. 2023;470:144387. https://doi.org/10.1016/j.cej.2023.144387
- [Google Scholar]
- Magnetic Fe3O4-c@MoS2 composites coordinated with peroxymonosulfate catalysis for enhanced tetracycline degradation. Journal of Alloys and Compounds. 2024;989:174318. https://doi.org/10.1016/j.jallcom.2024.174318
- [Google Scholar]
- A comprehensive review on the boosted effects of anion vacancy in the heterogeneous photocatalytic degradation, part i: Focus on sulfur, nitrogen, carbon, and halogen vacancies. Ecotoxicology and Environmental Safety. 2024;269:115927. https://doi.org/10.1016/j.ecoenv.2024.115927
- [Google Scholar]
- Biochar encapsulated metal nanoflowers for high efficient degradation of metronidazole via peroxymonosulfate activation. Separation and Purification Technology. 2024;328:125081. https://doi.org/10.1016/j.seppur.2023.125081
- [Google Scholar]
- Nitrogen doped bimetallic sludge biochar composite for synergistic persulfate activation: Reactivity, stability and mechanisms. Environmental Research. 2023;229:115998. https://doi.org/10.1016/j.envres.2023.115998
- [Google Scholar]
- A comprehensive review on the boosted effects of anion vacancy in the photocatalytic and photoelectrochemical water-splitting: Focus on oxygen vacancy. Materials Today Energy. 2025;48:101754. https://doi.org/10.1016/j.mtener.2024.101754
- [Google Scholar]
- Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT) Chemical Engineering Journal. 2020;395:125094. https://doi.org/10.1016/j.cej.2020.125094
- [Google Scholar]
- Silver and g-C3N4 co-modified biochar (Ag-CN@BC) for enhancing photocatalytic/PDS degradation of BPA: Role of carrier and photoelectric mechanism. Environmental Research. 2024;262:119972. https://doi.org/10.1016/j.envres.2024.119972
- [Google Scholar]
- Copper-doped orange peel biochar activated peroxydisulfate for efficient degrading tetracycline: The critical role of C-OH and Cu. Environmental Research. 2024;263:120265. https://doi.org/10.1016/j.envres.2024.120265
- [Google Scholar]
- Biochar-supported FeCo-MOF derivative catalyzes PDS-mediated degradation of tetracycline. Separation and Purification Technology. 2024;349:127841. https://doi.org/10.1016/j.seppur.2024.127841
- [Google Scholar]
- Visible-light-driven peroxydisulfate activation by biochar-loaded Fe-Cu layered double hydroxide for penicillin G degradation: Performance, mechanism and application potential. Environmental Research. 2024;263:120043. https://doi.org/10.1016/j.envres.2024.120043
- [Google Scholar]
- Unraveling the mechanism and the key role of amorphous structure C-O-Fe in tetracycline degradation by activation of persulfate in lotus leaf-based red mud-modified biochar. Chemical Engineering Journal. 2024;499:156078. https://doi.org/10.1016/j.cej.2024.156078
- [Google Scholar]
- MoO2-enhanced fe-loaded biochar promotes Fe2+/Fe3+ cycling for activation of peroxydisulfate to degrade organic pollutants. Environmental Technology & Innovation. 2024;35:103736. https://doi.org/10.1016/j.eti.2024.103736
- [Google Scholar]
- Nitrogen self-doped chlorella biochar as a peroxydisulfate activator for sulfamethazine degradation: The dominant role of electron transfer. Journal of Cleaner Production. 2024;440:140951. https://doi.org/10.1016/j.jclepro.2024.140951
- [Google Scholar]
- Pomelo peel biochar supported nZVI@BiO as a persulfate activator for the degradation of acetaminophen: Enhanced performance and degradation mechanism. Separation and Purification Technology. 2024;350:127966. https://doi.org/10.1016/j.seppur.2024.127966
- [Google Scholar]
- Degradation of tetracycline based on activated persulfate by microbial-induced calcium carbonate precipitation as templates modified sludge biochar. Journal of Water Process Engineering. 2024;60:105193. https://doi.org/10.1016/j.jwpe.2024.105193
- [Google Scholar]
- Optimization of product distribution during co-pyrolysis of furfural residues and waste tyres via response surface method and infrared heating. Separation and Purification Technology. 2025;354:129209. https://doi.org/10.1016/j.seppur.2024.129209
- [Google Scholar]
- A co-precipitation synthesized Zn(II)/Ni(II) magnetic heterostructure ferrite for photocatalytic degradation of metronidazole: Charge carriers transfer mechanism, kinetics and thermodynamic aspects. International Journal of Hydrogen Energy. 2025;106:1429-1442. https://doi.org/10.1016/j.ijhydene.2025.02.031
- [Google Scholar]
- Biochar activated by oxygen plasma for supercapacitors. Journal of Power Sources. 2015;274:1300-5. https://doi.org/10.1016/j.jpowsour.2014.10.169
- [Google Scholar]
- A comprehensive study on the removal of cd(II) from aqueous solution on a novel pentetic acid-clinoptilolite nanoparticles adsorbent: Experimental design, kinetic and thermodynamic aspects. Solid State Sciences. 2020;99:106071. https://doi.org/10.1016/j.solidstatesciences.2019.106071
- [Google Scholar]
- EDTA-functionalized clinoptilolite nanoparticles as an effective adsorbent for Pb(II) removal. Environmental Science and Pollution Research International. 2018;25:14043-14056. https://doi.org/10.1007/s11356-018-1461-0
- [Google Scholar]
- Efficient solid amino acid–clinoptilolite nanoparticles adsorbent for Mn(II) removal: A comprehensive study on designing the experiments, thermodynamic and kinetic aspects. Solid State Sciences. 2020;101:106124. https://doi.org/10.1016/j.solidstatesciences.2020.106124
- [Google Scholar]
- A comparison of adsorption capacity of several synthesis methods of cellulose-based absorbent towards Pb(II) removal: Optimization with response surface methodology. International Journal of Biological Macromolecules. 2023;253:127115. https://doi.org/10.1016/j.ijbiomac.2023.127115
- [Google Scholar]
- A kinetic and thermodynamic study of Cd(II) removal by hexylamine-clinoptilolite nanoparticles composite. Desalination and Water Treatment. 2018;116:158-169. https://doi.org/10.5004/dwt.2018.22604
- [Google Scholar]
- One-step synthesis of magnetic n-doped carbon nanotubes derived from waste plastics for effective Cr(Ⅵ) removal. Arabian Journal of Chemistry. 2024;17:105956. https://doi.org/10.1016/j.arabjc.2024.105956
- [Google Scholar]
- A comprehensive study on the kinetics and thermodynamic aspects of batch and column removal of Pb(II) by the clinoptilolite–glycine adsorbent. Materials Chemistry and Physics. 2020;240:122142. https://doi.org/10.1016/j.matchemphys.2019.122142
- [Google Scholar]
- An efficient modified zeolite for simultaneous removal of Pb(II) and Hg(II) from aqueous solution. Journal of Molecular Liquids. 2017;230:221-229. https://doi.org/10.1016/j.molliq.2017.01.029
- [Google Scholar]
- Modification of an iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. Journal of Colloid and Interface Science. 2015;440:272-281. https://doi.org/10.1016/j.jcis.2014.11.017
- [Google Scholar]
- Equilibrium and kinetic data, and adsorption mechanism for adsorption of lead onto valonia tannin resin. Chemical Engineering Journal. 2008;143:32-42. https://doi.org/10.1016/j.cej.2007.12.005
- [Google Scholar]
- One-pot synthesis of a magnetic Zn/iron-based sludge/biochar composite for aqueous Cr(VI) adsorption. Environmental Technology & Innovation. 2022;28:102661. https://doi.org/10.1016/j.eti.2022.102661
- [Google Scholar]
- Adsorption mechanism of Cr(VI) on woody-activated carbons. Heliyon. 2023;9:e13267. https://doi.org/10.1016/j.heliyon.2023.e13267
- [Google Scholar]
- Removal of aqueous oxytetracycline using copper-loaded biochar-activated peroxodisulfate: Synergistic mechanism of adsorption and nonradical 1O2. Journal of Environmental Chemical Engineering. 2024;12:114302. https://doi.org/10.1016/j.jece.2024.114302
- [Google Scholar]
- Role of radical and non-radical pathway in activating persulfate for degradation of p-nitrophenol by sulfur-doped ordered mesoporous carbon. Chemical Engineering Journal. 2020;384:123304. https://doi.org/10.1016/j.cej.2019.123304
- [Google Scholar]
- A visible light driven AgBr/G-C3N4 photocatalyst composite in methyl orange photodegradation: Focus on photoluminescence, mole ratio, synthesis method of G-C3N4 and scavengers. Composites Part B: Engineering. 2020;183:107712. https://doi.org/10.1016/j.compositesb.2019.107712
- [Google Scholar]
- Upcycling waste plastics into FeNi@CNTs chainmail catalysts for effective degradation of norfloxacin: The synergy between metal core and CNTs shell. Separation and Purification Technology. 2023;326:124735. https://doi.org/10.1016/j.seppur.2023.124735
- [Google Scholar]
- Synergistic scavenging of sulfamethoxazole and hexavalent chromium in groundwater sample induced by iron-char composites activated persulfate triggering dominant electron transfer process. Chemical Engineering Journal. 2024;497:154911. https://doi.org/10.1016/j.cej.2024.154911
- [Google Scholar]







